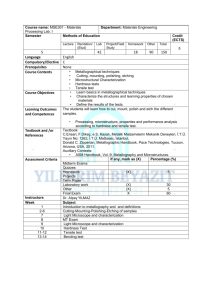
Lab 1 Assignment

THERMAL TREATMENT PROCESSES
ME 311: Manufacturing Processes Course
Laboratory
1
Objectives:
Learn to use Rockwell hardness machine to measure hardness of different specimens.
Understand the processes and effects of annealing and quenching on different specimens.
1
Section I: Introduction to Thermal Treatment Processes
A metallic material can be altered via mechanical and/or thermal means to change the mechanical properties of the material. Such treatments are most often used to alter strength, hardness, and ductility of a material.
1. Heat Treatment: Tempering of Hardened Steel
The heat treatment process for steel and steel alloys is composed of three steps: a) Heating to a temperature at which austenite is formed (austenitizing) b) Rapid cooling (quenching) c) Reheating to stabilize structure (tempering)
Austenite is a solid solution of carbon in iron in a face centered cubic (FCC) crystalline structure which is stable at elevated temperature. The temperature at which austenite formation occurs depends primarily on carbon content of the steel. This temperature can be determined from the phase equilibrium diagram for the particular steel composition. The material must be held at the austenitizing temperature for a period of time to ensure completeness of the phase transformation and homogeneous structure. The amount of time required is dependent on the size and shape of the work piece as well as its composition.
After the time required for austenite formation, the material is rapidly cooled by quenching. Most often, quenching is accomplished by immersing the material in oil or water although air quenching is also used. Under conditions of rapid cooling, austenite transforms into an unstable (non equilibrium) phase known as martensite . This phase is a supersaturated solution of carbon in iron in a body centered tetragonal structure. Martensite is very hard, relatively brittle phase which provides the ability of strengthening steel to very high levels. Quenching usually results in a structure composed of martensite plus ferrite (solid solution of carbon in iron in body centered cubic structure) and iron carbide (cementite). The proportions present after quenching depend on carbon content and cooling rate. Higher carbon content and rapid cooling tend to produce larger proportions of martensite.
Martensite is hard and brittle. In order to reduce brittleness, increase ductility, and relieve internal stresses from rapid cooling step, the material is subjected to a second heating operation known as tempering . The temperature for the tempering step must be below the austenite transformation temperature and is usually between 400F and 800F. The resultant properties of the steel after tempering depend on the time allowed for tempering as well as the temperature. In this lab, a constant temperature will be used and the effect of tempering time on hardness will be investigated.
2
Figure 1: Iron-Carbon Phase Equilibrium diagram. ( Source: Material Science and Metallurgy, 4 th edition, Pollack, Prentice-Hall 1988 )
2. Hardenability: The Jominy Test
The Jominy end quench test is the most commonly used method for determining the hardenability of steel. Hardenability is the relative capacity of steel to be hardened by transformation to martensite. In this test, a standard specimen (octagonal cross-section bar stock) is heated to the austenite range and quenched by impinging a stream of cold water on one end of the specimen while the specimen is held in a vertical orientation. This procedure results in cooling rates ranging from very rapid at the end impinged by the water to very slow at the opposite end. With the variation in cooling rate along the length, a single specimen will be composed of material ranging from hard martensite to soft pearlite.
The effect of cooling rate on the resultant structure of steel can be observed on a time-temperature transformation diagram. A sample diagram is provided (Figure 2) that shows three cooling rates resulting in cooling curves A , B , and C . Curve A represents conditions at the end of the Jominy specimen nearest the water stream. The material at this end will be composed primarily of
3
martensite and should be relatively hard. Curve C is indicative of conditions farther along the bar and indicates a softer composition which includes ferrite and pearlite.
The effect of cooling rate is evidenced by obtaining hardness measurements along the length of the bar. A plot of hardness versus distance (Figure 3) is the standard method for presenting hardenability data.
Figure 2: Effect of cooling rate on hardness
Figure 3: Effect of distance on hardness
4
Name: Section:_ Group:_ Due Date:
Section II: Heat Treatment Exercise
The exercises of laboratory 2 investigate heat treatment techniques and measurement of hardness of different specimen.
The instructor/TA will demonstrate heat treatment process and use of Rockwell hardness machine.
A. Steel Heat Treatment
The instructor/TA will provide samples of AISI 1080 steel. These samples were first heated to a temperature of 1700F (Austenizing temperature for steel). One sample was quenched in water and another in oil. For each sample, remove the scale formed during heat treatment using sand paper or emery cloth. Using Rockwell tester, obtain and record five Rockwell C hardness measurements on the polished and unpolished surface of each sample. Record these readings in
Table 1.
1
2
3
4
5
Table 1: Hardness Rockwell C for polished and scaled surfaces for heat treated AISI 1080 Steel
Measurement Water quenched Oil quenched
Number Polished Scaled Polished Scaled
The instructor will provide different samples from the above experiment that were placed in the furnace preheated to 650F for 5 min, 10 min, 20 min and 30 min. These samples were then allowed to cool in air. Measure the Rockwell C hardness on the cut surface of each sample and record in Table 2.
1
2
3
4
5
Table 2: Hardness vs Tempering Time for AISI 1080 Steel austenized at 1700F and then tempered at
650F
Measurement Water Quenched Oil number
Initial
Quenched
5 mins 10 mins 20 mins 30 mins 30 mins
B. Jominy Test
An AISI 1040 or 4140 alloy specimen will be given with indentations along the length. Use the
Rockwell hardness machine to measure Rockwell hardness values. Each measurement should be made in the center of the flat area. Record the data in Table 3. Note: One person should perform the hardness measurement while another person holds the specimen steady in position
5
on the machine. This will avoid erroneous reading caused by the overhanging weight of the specimen.
Table 3: Hardness vs distance, Jominy test
Distance from end
Hardness
1040 Steel 4140 Steel
1/16”
57 58
1/8”
3/16”
1/4”
55
55
56
57
56
54
5/16”
3/8”
55
52
57
56
7/16”
52 54
1/2”
9/16”
5/8”
48
49
50
52
53
53
11/16”
3/4”
13/16”
48
45
43
49
47
46
7/8”
15/16”
43
41
43
42
1”
39 40
Distance from end
1-1/8”
1-1/4”
1-3/8”
1-1/2”
1-5/8”
1-3/4”
1-7/8”
2”
2-1/4”
2-1/2”
2-3/4”
3”
3-1/4”
3-1/2”
3-3/4”
4”
Hardness
1040 Steel
38
36
36
33
33
33
32
31
34
32
32
31
31
31
30
27
4140 Steel
40
39
37
36
34
36
34
33
33
33
32
34
32
31
31
30
Using the data obtained and recorded in the tables above, prepare a report which answers the following questions.
1. What effect does surface scale have on hardness measurements ?
2. How does tempering time affect the hardness of AISI 1080 steel ?
3. What is the effect of cooling rate on hardness of 1040 and 4140 steel per the Jominy test ?
In support of your answers to these questions, include graphs as follows: a) Mean hardness vs tempering time for water quenched 1080 steel b) Hardness vs distance for both Jominy tests c) Bar graph of Table 1 data showing 95% confidence intervals for each condition. Compute mean hardness, sample standard deviation for each condition. Use small sample statistical methods (n=5) and present in tabular for such as shown below. Show your calculations.
Treatment
Water-polished
Water-scale
Oil-polished
Oil-scaled
Mean Hardness Standard Deviation 95% confidence limits
6