EXPERIMENT G mod
advertisement

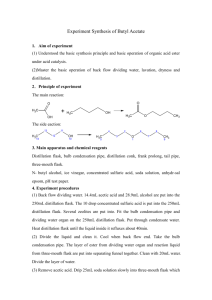
EXPERIMENT G Oil of Cloves - Isolation and Derivatization of Eugenol NOTE: As you work on Experiment G, refer to the Flow Diagrams in your notebook. PROCEDURE PART I: Isolation and Purification of Eugenol Check out: 500mL thermowell and heat-resistant gloves (both must be returned on the same day) The distillation of clove oil requires a full lab period and must be completed in one day. Consequently, you must have your prelab notebook approved before the lab period in which you will distill your clove oil. Obtain a jar of whole cloves (approx. 10g) after your prelab notebook is approved. Using the coffee grinder, process the cloves for no more than 30 seconds. The ground cloves should be coarse solid, not a fine powder. Record the weight of your ground cloves and place them in a 500mL round bottom flask using a power funnel. Add approx. 250mL hot water. Assemble the co-distillation apparatus (see Figure at end of this document). Use the ring stands, not the "monkey bars". Note the heating mantle must be supported by a ring stand. Also, note the position of clamps and be careful to not leave any portion unsupported so it does not fall and break. You may want to ask your instructor to check your apparatus before you turn on the thermowell. [See below for a "step by step" set of instructions to set up your codistillation] Turn your thermowell setting to about 5 (or 50). After a short while, you should observe bubbling and vapors rising in the apparatus, followed by dripping of liquid into your 250mL flask (appearance?). Periodically record the time and temperature of the distillate at your stillhead. As a general rule, keep the water level in your 500mL flask at about the level of the top of the thermowell. When it drops below this level, add hot water slowly using the separatory funnel. If your distillation rate is slow, the instructor may wrap part of your apparatus with foil to insulate it. Each thermowell is different and your specific location in the lab may affect the distillation rate. While your distillation is in progress, do not leave it unattended at any time. If you must leave (e.g. to go to the bathroom), turn off the power to your thermowell. Distill until you have approximately 150mL in your Erlenmeyer flask. Cover your flask with Duraseal + a rubber band and leave in your drawer. Disposal: Using the clamp as a handle for your 500mL flask, pour the remaining water + clove solid into the marked plastic bottles in the hood (DO NOT pour down the drain!). If you need to rinse your flask, wait a few minutes to let it cool, then rinse with tap water (hot glass will crack if cold water is used). In the next lab period, note the appearance of your crude clove oil in your notebook. Extract the crude clove oil with two 40mL portions of hexanes. Combine the organic layers. Set aside about 1-2mL of this crude clove oil in a labelled test tube for later IR analysis (see below). Place the combined hexane extracts in a 250mL separatory funnel (check out from Stockroom) and extract with two 30mL portions of 1M NaOH (aqueous). Combine the two NaOH extracts (which contain the sodium salt of eugenol) and add at least 20mL of 3M HCl (aqueous) until pH paper indicates a pH of ~2 has been reached (may have to add a little more in some cases). Extract this acidified solution in a 125mL separatory funnel with two 30mL portions of hexanes. Combine the organic extracts in a 125mL Erlenmeyer flask and add anhydrous magnesium sulfate to remove traces of water. Gravity filter into a weighed 100mL round bottom flask. Rinse the magnesium sulfate with 5-10mL of hexanes and filter into the flask. Using the rotary evaporator, evaporate the hexanes. The resulting purified eugenol should be clear and colorless (resembling water). If it is cloudy, you may have to repeat the drying step by adding hexanes and magnesium sulfate, filter, then evaporate. Record the weight of your flask + eugenol and calculate your recovery of eugenol. IR Analysis of Crude Clove Oil and Purified Eugenol: For the Crude Clove Oil, perform the same drying steps using hexanes and magnesium sulfate as described for eugenol. After filtration, evaporate the hexanes. Place the flask containing your sample under vacuum for an additional 10min or more to ensure traces of solvent are removed. Measure the IR of the crude clove oil and purified eugenol. GC Analysis of Crude Clove Oil and Purified Eugenol: This will be provided by your instructor and will be available in your Chem 113A Canvas Files. Print this out and include in your report. Experiment G Part I - Important Notes for Codistillation ON THE DAY WHEN THE CLASS BEGINS CLOVE DISTILLATION, I WILL NOT CHECK NOTEBOOKS UNTIL AFTER EVERYONE COMPLETES THEIR DISTILLATION SETUP. The distillation requires the entire lab period (start to finish), so if your notebook is not checked before the lab period begins, you will not be able to do the distillation until the next lab period. Plan ahead!! On the day of the distillation, do these steps in order as soon as you get to lab: 1) put ~250mL distilled water in a 600mL beaker on LOW steam to heat while you set up the apparatus. 2) Check out a 500mL thermowell 3) Grind the cloves in your jar (do not weigh yet) - 30 SECONDS ONLY 4) Weigh your ground cloves 5) Put ground cloves in your 500mL round bottom flask using a powder funnel 6) Add ~ 250mL of the heated water you prepared (does not have to be boiling hot) 7) Clamp the flask in the thermowell and assemble distillation apparatus; LIGHTLY grease ALL joints! Follow the remaining procedure as described. WHILE THE DISTILLATION IS IN PROGRESS, YOU MUST STAY BY YOUR APPARATUS AT ALL TIMES!! Experiment G - Apparatus for Clove Oil Distillation thermometer Use 2 ringstands (not monkeybars) do not stopper Spring clamps (2) warm water to replace distillate water out water in spring clamp clamp clamp (do not tighten!) clamp raise thermowell using ring clamp 250mL flask PROCEDURE PART II: Aryloxyacetic acid Derivative of Eugenol NOTE: The following procedure is written for 1.0g of purified Eugenol. If you have <1.0g from Part I, check with your instructor. In a 125mL Erlenmeyer flask, add 1.0g of purified Eugenol, followed by 5 mL of 33% NaOH (aqueous). (Note any change in appearance in this and subsequent steps) Swirl, then add 2.5mL of 50% chloroacetic acid (aqueous ClCH2COOH). If the solids do not dissolve completely, add from 1 to 5 mL of additional water with gentle heat if necessary (HOWEVER, do not exceed adding more than 5mL, even if some solid remains). Using gentle steam, heat the contents on a steam bath for 30 min. [This is a good stopping point if you are near the end of a lab period. Stopper your flask and leave in the hood with flasks from your section] Cool the flask for a few minutes and add 10 mL of DI water. While swirling, add 3M HCl in small portions (usually 5 - 10mL HCl). A precipitate should form. Dip a glass rod in the solution and touch to pH paper to verify that the pH is 2 or less. Transfer the solution to a 125mL separatory funnel and extract with 35mL of ethyl acetate. Separate the organic and aqueous phases (make sure you know which is which!) Extract the organic phase with 35mL sodium bicarbonate (aqueous). Separate the organic and aqueous phases. Transfer the aqueous phase to a 125 mL Erlenmeyer flask. With vigorous stirring, carefully add 3M HCl in portions until the pH reads 2 or less (approx. 10 to 20mL HCl). The crude derivative should precipitate. Isolate the crude derivative by vacuum filtration. Let the solid sit under vacuum for 10-15 min to remove as much water as possible. Record the weight of the crude derivative in your notebook. Dissolve the crude derivative in 10mL of ethyl acetate in a 50 mL Erlenmeyer flask. Dry over anhydrous magnesium sulfate for 5-10 min. Gravity filter into a weighed 50mL round bottom flask. Rinse the magnesium sulfate with 5mL of ethyl acetate and filter into the round bottom flask. Evaporate the solvent using the rotary evaporator. Put the flask under vacuum for another 10-15min. The final aryloxyacetic acid derivative of Eugenol should be a clear and colorless solid. If not, you should recrystallize it as described below. When you have a clear, colorless solid, record the final weight of your eugenol derivative. Scrape as much as possible into a clean vial and label. Measure the melting point of your derivative, which should be 99 to 100oC. Hot Toluene Recrystallization. To the flask containing the impure derivative, add 10mL toluene and dissolve using gentle heat on the steam cone. Remove from heat and allow to cool gradually to room temperature. Collect the crystals by vacuum filtration, rinsing with a few mL of hexanes. Allow air to pass over the solid for 10-15min before measuring the melting point. If solid does not appear after about 30min, add petroleum ether until a slight cloudiness appears and chill again to induce crystallization. Follow the steps as described above to collect the derivative crystals. [See the "writeup" section on the Chem 113A website for information on how to present your data and analysis in your report]