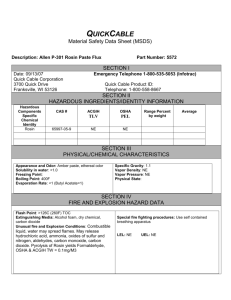
Scientific Opinion on the safety of glycerol esters - EFSA
advertisement

EFSA Journal 2010; 8(7):1654 SCIENTIFIC OPINION Scientific Opinion on the safety of glycerol esters of gum rosin for the proposed uses as a food additive1 EFSA Panel on Food Additives and Nutrient Sources added to Food (ANS)2, 3 European Food Safety Authority (EFSA), Parma, Italy ABSTRACT The Panel on Food Additives and Nutrient Sources added to Food provides a scientific opinion evaluating the safety of glycerol esters of gum rosin (GEGR) for the proposed use as a stabilising and emulsifying food additive in certain beverages to a maximum level of 100 mg/l. The Joint FAO/WHO Expert Committee on Food Additives established a group ADI of 0-25 mg/kg bw/day for glycerol esters of wood rosin (GEWR) and GEGR and tentative specifications for GEGR pending the submission of additional data. GEGR are obtained by esterification of refined gum rosin and are described as a complex mixture of tri- and diglycerol esters of resin acids and a residual fraction of monoglycerol esters. Besides these esters, up to 4.2% free resin acids and up to 20.1% other non-acidic saponifiable and unsaponifiable substances are present in GEGR. The percentages of resin acids derived from the three fractions upon saponification of GEGR are known. However, data on the identity and quantity of individual components in the three fractions of GEGR are absent and also data on the proportions of glycerol monoesters, (1,2)-glycerol diesters, (1,3)-glycerol diesters and glycerol triesters are not provided. In view of missing toxicity studies for GEGR, analytical data were submitted to demonstrate that GEGR are chemically equivalent to GEWR which have already been authorised as a food additive by Directive 95/2/EC. Overall, the Panel concluded that the chemical and toxicological characterisation of GEGR is not adequate. The Panel also did not have sufficient information to evaluate the chemical equivalence of GEGR and GEWR on the basis of which the toxicological data obtained with GEWR could be used for read across. Therefore, the Panel concluded that the available data are too limited to conclude on the safety of GEGR as a food additive at the proposed uses and use levels. KEY WORDS Glycerol esters of gum rosin, glycerol-modified gum rosin, CAS Registry Number 8050-31-5. 1 On request from the European Commission, Question No EFSA-Q-2009-00450, adopted on 23 June 2010. 2 Panel members: F. Aguilar, B. Dusemund, P. Galtier, J. Gilbert, D.M. Gott, S. Grilli, R. Gürtler, J. König, C. Lambré, J-C. Larsen, J-C. Leblanc, A. Mortensen, D. Parent-Massin, I. Pratt, I.M.C.M. Rietjens, I. Stankovic, P. Tobback, T. Verguieva, R.A. Woutersen. Correspondence: ans@efsa.europa.eu 3 Acknowledgement: The Panel wishes to thank the members of the ANS Working Group B on Food Additives and Nutrient Sources added to Food for the preparation of this opinion: M. Bakker, D. Boskou, B. Dusemund, D. Gott, T. Hallas-Møller, A. Hearty, J. König, D. Marzin, D. Parent-Massin, I.M.C.M. Rietjens, G.J.A. Speijers, P. Tobback, T. Verguieva, R.A. Woutersen. Suggested citation: EFSA Panel on Food Additives and Nutrient Sources added to Food (ANS)Scientific Opinion on the safety of glycerol esters of gum rosin for the proposed uses as a food additive EFSA Journal 2010; 8(7):1654. [31 pp.]. doi:10.2903/j.efsa.2010.1654. Available online: www.efsa.europa.eu © European Food Safety Authority, 2010 1 The use of glycerol esters of gum rosin as a food additive SUMMARY Following a request from the European Commission to the European Food Safety Authority (EFSA), the Scientific Panel on Food Additives and Nutrient Sources added to Food (ANS) was asked to provide a scientific opinion on the safety of glycerol esters of gum rosin (GEGR) when used as a stabilising and emulsifying food additive in certain non-alcoholic flavoured cloudy drinks and certain cloudy spirit drinks to a maximum level of 100 mg/l. GEGR have been evaluated by the Joint FAO/WHO Expert Committee on Food Additives (JECFA) in 2009. The Committee established a group ADI of 0 – 25 mg/kg bw/day for GEWR (glycerol esters of wood rosin) and GEGR. The specifications for GEGR were made tentative, pending the submission of additional data and information by the end of 2010 concerning the identity of GEGR, the resin acid composition and methods to identify the individual glycerol esters of rosins and their differentiation. Furthermore, the Committee requested the full reports of the two 90-day toxicity studies in rats performed using oral administration of GEGR, to confirm the validity of the comparison of GEWR with GEGR. GEGR are obtained by esterification of refined gum rosin originating, according to the petitioner, from Pinus oocarpa Schiede. GEGR are described as a complex mixture of tri- and diglycerol esters of resin acids from gum rosin, with glycerol triabietate as the main component and a residual fraction of monoglycerol esters. Besides the glycerol esters of resin acids [fraction (a)] which amount only to 75.7 78.2% of GEGR, 2.8 - 4.2% free resin acids [fraction (b)] and 19 - 20.1% other non-acidic saponifiable and unsaponifiable substances [fraction (c)] are present in GEGR according to the analysis of 3 samples of GEGR, the results of which were provided by the petitioner upon request from the Panel. The percentages of resin acids derived from fractions (a), (b) and (c) after saponification of GEGR are known. Abietic acid, dehydroabietic acid and communic acid are the main representatives. The Panel noted, however, that data on the identity and quantity of individual components in fractions (a), (b) and (c) of unsaponified GEGR are missing and also data on the proportions of glycerol monoesters, (1,2)-glycerol diesters, (1,3)-glycerol diesters and glycerol triesters in fraction (a) are not provided. According to the petitioner, the non-acidic fraction of the source material gum rosin, which is presumed to be at least partly retained during GEGR production and to result in fraction (c), is composed of natural esters of resin acids, esters of fatty acids and of unsaponifiables. As described in published literature, the unsaponifiables in gum rosin comprise more than 40 components, e.g. volatile monoterpenes, diterpene alcohols and diterpene aldehydes, the latter two groups being described as “non volatiles”. The petitioner states that volatile organic compounds of the non-acidic fraction of gum rosin will be stripped off in the manufacturing procedure of GEGR. The Panel noted that, according to the description of the petitioner, the gum rosin used as source in the production of GEGR meets the definition of the wood rosin as source of GEWR laid down in the Commission Directive 2008/84/EC4 as far as the content of approximately 10% neutrals in the refined gum rosin is concerned. But the Panel also noted that a high content of fraction (c) in GEGR of up to 20% results after the esterification and final purification. This implies that the percentage of the unknown substances of fraction (c) is twice the percentage of neutrals in the refined source material. According to the petitioner, this increase is due to the new formation of unsaponifiables during the process of esterification at high temperature in the presence of oxygen. The composition of fraction (c) is unknown. The possible occurrence of “non volatile” diterpenic substances (e.g. alcohols, aldehydes, esters) in larger amounts and of 3,5-dimethoxystilbene in fraction (c) has to be taken into consideration. The extent to which compounds of different volatility are removed in the course of manufacturing is unknown. 4 Commission Directive 2008/84/EC of 27 August 2008 laying down specific purity criteria on food additives other than colours and sweeteners EFSA Journal 2010; 8(7):1654 2 The use of glycerol esters of gum rosin as a food additive Physical and chemical parameters in the specification of GEGR are equivalent to those defined by the Commission Directive 2008/84/EC for GEWR limiting e.g. the concentration of free acids in GEGR by the acid value. The Panel noted that setting limits for glycerol monoesters of resin acids, which are supposed to undergo partial hydrolysis in the gastrointestinal tract, and furthermore information on the levels of fraction (b) and fraction (c) might be relevant for the specifications. The Panel noted that for GEGR no studies are available on: i) absorption, distribution, metabolism and excretion, ii) short-term and subchronic toxicity (the results of two 90-day toxicity studies in rats fed GEGR were submitted only as short summaries), iii) genotoxicity, iv) chronic toxicity and carcinogenicity, v) reproductive and developmental toxicity. Considering free resin acids present as fraction (b) in GEGR, selected data on absorption, distribution, metabolism and excretion and on genotoxicity as reviewed in a previous JECFA monograph are available. The Panel noted that there are no data from in vivo genotoxicity testing to overrule positive in vitro findings. In view of the limited toxicity data available for GEGR, the petitioner submits analytical data to demonstrate that GEGR are chemically equivalent to GEWR (E 445). On this basis, the petitioner claims that the toxicity data for GEWR are directly relevant to GEGR. GEWR have already been authorised by Directive 95/2/EC5, allowing their use as a stabiliser and emulsifier in non-alcoholic flavoured cloudy drinks and certain cloudy spirit drinks up to a maximum level of 100 mg/l. Physical and chemical properties were measured in six lots of GEGR and five samples of GEWR. Infrared (IR) Spectroscopy, Gas Chromatography (GC), and Nuclear Magnetic Resonance (NMR) were used to analyse the products. The results demonstrate that the two products are similar. The percentages of individual resin acids obtained after saponification of GEGR or GEWR are also in the same range. Some difference is observed for the percentages of fraction (c). Taking into account the results of different analyses showing some batch-to-batch variations for GEGR, the values for fraction (c) range from 15.6 to 20.1% for GEGR and 12.5 to 13% for GEWR. According to the petitioner, currently there are no qualitative and quantitative data available concerning the individual components in fractions (a), (b), and (c) of GEGR and GEWR as a basis for comparison. The Panel noted that the concentrations of individual resin acids measured in GEWR and GEGR samples after saponification, covering the fraction of free resin acids [fraction (b)], the resin acids derived from glycerol esters [fraction (a)] and the acids derived from esters of fraction (c), are very similar. But apart from the missing qualitative and quantitative analysis of individual glycerol mono- diand triesters in fraction (a) of GEGR and GEWR, most notably the components of fraction (c) of GEGR and GEWR, which may differ due to different manufacturing procedures and to different botanical sources, are unknown. Since the content of fraction (c) in GEGR accounts for up to 20%, this information is most relevant for the evaluation of the equivalence of GEGR and GEWR. The Panel could not conclude, based on the chemical data provided, that GEGR and GEWR are chemically equivalent. Therefore, the Panel could not base the safety evaluation of GEGR on the results of the toxicological studies available for GEWR. Dermal contact sensitisation is seen with different types of rosins and modified rosins and their components, as well as with GEGR and 1-glycerol monoabietate. Since from ingestion of glycerol esters of rosins with beverages, allergic responses have not been reported, the Panel did not consider it likely that the oral exposure via the intended use and use levels of GEGR in beverages is associated with a 5 European Parliament and Council Directive 95/2/EC of 20 February 1995, on food additives other than colours and sweeteners EFSA Journal 2010; 8(7):1654 3 The use of glycerol esters of gum rosin as a food additive relevant risk of adverse effects for individuals with a known contact hypersensitivity to rosin or modified rosin products. The exposure to GEGR was calculated based on the information available from the EFSA Concise Database in Exposure Assessment. The average exposure would range in Europe from 0.07 to 0.74 mg/kg bw/day and at the 97.5th percentile from 0.5 to 3.3 mg/kg bw/day. The Panel also calculated the exposure to GEGR based on the UK data for children using the intake of non-alcoholic flavoured drinks and the intended use level of 100 mg/l. The exposure was estimated to be 1.7 mg/kg bw/day on average and 5.8 mg/kg bw/day at the 97.5th percentile. The Panel noted that these estimates are conservative, since GEGR is not intended to be added to all non-alcoholic flavoured drinks but to citrus-fruit based drinks only. The Panel concluded that the chemical and toxicological characterisation of GEGR is not adequate and that the absence of toxicological study reports on GEGR prevents the evaluation of the safety of GEGR. The Panel also concluded that there is not sufficient information to evaluate the chemical equivalence of GEGR and GEWR, and that therefore the toxicological data obtained with GEWR could not be used for read across to GEGR. The Panel concluded that the available data are too limited to conclude on the safety of GEGR as a food additive at the proposed uses and use levels. EFSA Journal 2010; 8(7):1654 4 The use of glycerol esters of gum rosin as a food additive TABLE OF CONTENTS Abstract ....................................................................................................................................................1 Summary ..................................................................................................................................................2 Table of Contents .....................................................................................................................................5 Background as provided by the European Commission...........................................................................6 Terms of reference as provided by the European Commission ................................................................6 Assessment ...............................................................................................................................................7 1. Introduction .....................................................................................................................................7 2. Technical data ..................................................................................................................................7 2.1. Identity of the substance .........................................................................................................7 2.2. Specifications ........................................................................................................................11 2.3. Manufacturing process ..........................................................................................................12 2.4. Methods of analysis in food ..................................................................................................13 2.5. Stability, reaction and fate in food ........................................................................................13 2.6. Equivalence of GEGR and GEWR .......................................................................................13 2.7. Case of need and proposed uses............................................................................................16 2.8. Information on existing authorisations and evaluations........................................................16 2.9. Exposure ...............................................................................................................................18 3. Biological and toxicological data ..................................................................................................18 3.1. Absorption, distribution, metabolism and excretion .............................................................19 3.1.1.Glycerol esters of gum rosin .................................................................................................19 3.1.2.Free resin acids - Fraction (b) ...............................................................................................19 3.2. Toxicological data .................................................................................................................20 3.2.1.Acute oral toxicity ................................................................................................................20 3.2.2.Short-term and subchronic toxicity ......................................................................................20 3.2.3.Genotoxicity .........................................................................................................................20 3.2.3.1. Glycerol esters of gum rosin ....................................................................................20 3.2.3.2. Free resin acids - Fraction (b) ..................................................................................20 3.2.4.Chronic toxicity and carcinogenicity ....................................................................................20 3.2.5.Reproductive and developmental toxicity ............................................................................20 3.2.6.Other studies/Contact sensitisation .......................................................................................20 4. Discussion ......................................................................................................................................21 Documentation provided to EFSA .........................................................................................................26 References ..............................................................................................................................................26 Glossary/Abbreviations ..........................................................................................................................31 EFSA Journal 2010; 8(7):1654 5 The use of glycerol esters of gum rosin as a food additive BACKGROUND AS PROVIDED BY THE EUROPEAN COMMISSION Emulsifiers and stabilisers are a functional class of food additives which are regulated under Directive 95/2/EC of the European Parliament and of the Council on food additives other than colours and sweeteners. Directive 95/2/EC allows the use of Glycerol Esters of Wood Rosin (E 445) in non alcoholic flavoured cloudy drinks and certain cloudy spirit drinks to a maximum level of 100 mg/l. Glycerol Esters of Wood Rosin are a complex mixture of tri and diglycerol esters of resin acids obtained from wood rosin, a product of natural origin derived from the aged stumps of pine trees. They are lipophilic in nature and combine with the oil component of oil in water emulsions that produce the cloudy appearance of some drinks, increasing the density of the oil and thereby stabilising the emulsion and maintaining the cloudy appearance of the drink for longer. A manufacturer of Glycerol Esters of Gum Rosin has made a request for the authorisation of Glycerol Esters of Gum Rosin to be used in certain types of drink. The manufacturer states that the component resin acids in the gum rosin, which is derived from living pine trees are the same as those found in the wood rosin which is derived from aged pine stumps and that the specifications of purity for the two substances are identical. Similarly, the technological function of the Glycerol Esters of Gum Rosin is equivalent to that of the authorised food additive. TERMS OF REFERENCE AS PROVIDED BY THE EUROPEAN COMMISSION In accordance with Article 29 (1) (a) of Regulation (EC) No 178/2002, the European Commission asks the European Food Safety Authority to provide a scientific opinion on the safety of Glycerol Esters of Gum Rosin as a stabiliser and emulsifier in certain types of drink. EFSA Journal 2010; 8(7):1654 6 The use of glycerol esters of gum rosin as a food additive ASSESSMENT 1. Introduction The present opinion deals with the safety of glycerol esters of gum rosin (GEGR) when used as a food additive with the function of a stabiliser and emulsifier in certain types of drinks. Due to the limited toxicity data available for GEGR, the petitioner submitted analytical data to demonstrate that GEGR are chemically equivalent to glycerol esters of wood rosin (GEWR, E 445). On this basis, the petitioner claims that toxicity data for GEWR are directly relevant for GEGR. GEWR have already been authorised by Directive 95/2/EC6, allowing their use as a stabiliser and emulsifier in non-alcoholic flavoured cloudy drinks and certain cloudy spirit drinks up to a maximum level of 100 mg/l. GEGR have been evaluated by JECFA in its 71st meeting in 2009. JECFA established a group ADI of 0 - 25 mg/kg bw/day for GEWR and GEGR and asked for the missing full reports of the two 90-day toxicity studies with GEGR and analytical information concerning its tentative specifications (JECFA, 2010). 2. Technical data 2.1. Identity of the substance GEGR are described by the petitioner as a complex mixture of tri- and diglycerol esters of resin acids from gum rosin, with a residual fraction of monoglycerol esters. The petitioner specified that Pinus oocarpa Schiede is the origin of the refined gum rosin used in the production of GEGR. According to the petitioner, refined gum rosin generally contains approximately 90% resin acids and approximately 10% neutrals (non-acidic substances). The resin acid fraction of gum rosin is a complex mixture of isomeric diterpenoid monocarboxylic acids with the empirical molecular formula of C20H30O2 and contains mainly abietic acid. Because gum rosin is derived from a natural source, use of different species and environmental factors (e.g., temperature, soil condition, etc.) can affect the amounts of the individual resin acids. The Chemical Abstracts Services (CAS) Registry Number 8050-31-5 is assigned to the glycerol esters of resin acids and is not unique to GEGR but also used for GEWR and glycerol esters of tall oil rosin (GETOR). GEGR are also referred to as glycerol-modified gum rosin. Rosin glycerol ester, glycerol abietate, glycerol triabietate, and ester gum are unspecific synonyms for GEGR, because they may also refer to other rosin glycerol esters such as GEWR and GETOR. GEGR are hard, yellow to pale amber-coloured thermoplastic solid resins. GEGR are soluble in acetone, aromatic and aliphatic hydrocarbons, terpenes, esters, ketones, citrus oil, and most other essential oils. GEGR are insoluble in lower-weight alcohols and water. According to the petitioner, the main component of GEGR is glycerol triabietate, but besides triesters other esters may also be formed as a result of incomplete esterification as reported by Gaefvert et al., (1994). These authors identified five compounds after esterification of abietic acid with glycerol under experimental conditions: abietic acid (unreacted), glycerol triabietate, 1,2-glycerol diabeitate, 1,3glycerol diabietate, and 1-glycerol monoabietate (Figure 1). Gaefvert et al. (1994) also conducted an analysis of glycerol-esterified Portuguese gum rosin. They found concentrations of abietic acid and 1glycerol monoabietate equal to 0.3% and 2.8%, respectively. 6 European Parliament and Council Directive 95/2/EC of 20 February 1995, on food additives other than colours and sweeteners EFSA Journal 2010; 8(7):1654 7 The use of glycerol esters of gum rosin as a food additive Glycerol triabietate 1,3-Glycerol diabietate Figure 1: 1,2-Glycerol diabietate 1-Glycerol monoabietate Products formed from esterification of abietic acid with glycerol (according to Gaefvert et al., 1994) Besides abietic acid, resin acids esterified with glycerol in GEGR also include dehydroabietic acid, palustric acid, neoabietic acid, pimaric acid, isopimaric acid, sandaracopimaric acid, communic acid, small fractions of tetrahydropimaric acid and dihydrodextropimaric acid, and some unspecified acids (Figure 2, Table 3). Abietane type: Abietic acid EFSA Journal 2010; 8(7):1654 Neoabietic acid 8 The use of glycerol esters of gum rosin as a food additive Palustric acid Dehydroabietic acid Pimarane type: Pimaric acid Isopimarane type: Isopimaric acid Sandaracopimaric acid Labdane type: Communic acid Figure 2: Resin acids found after saponification of GEGR EFSA Journal 2010; 8(7):1654 9 The use of glycerol esters of gum rosin as a food additive Results from three analysed samples presented by the petitioner upon request from the Panel showed that GEGR contain: 75.7 - 78.2% glycerol esters of resin acids (mono-, di- and tri-esters), thereafter called fraction (a), 2.8 - 4.2% free resin acids, thereafter called fraction (b), 19 - 20.1% other substances described as unsaponifiables, thereafter called fraction (c), The Panel noted that the term “unsaponifiables” does not sufficiently describe this fraction, e.g. this fraction could contain esters of fatty acids which are saponifiables. The petitioner did not provide data on the identity and quantity of individual components in fractions (a), (b) and (c). The petitioner also did not provide data on the proportions of glycerol monoesters, (1,2)-glycerol diesters, (1,3)-glycerol diesters and glycerol triesters in fraction (a). Concerning glycerol monoesters of resin acids, the petitioner claimed that they are only present in very small amounts in GEGR. This would be supported by the comparison of NMR spectra between GEGR and GEWR showing that GEGR has an equal or higher degree of esterification than GEWR and by the molecular weight distribution of two GEGR samples determined by Size Exclusion Chromatography. The Panel noted that the level of the glycerol monoesters of resin acids in GEGR has not been specified. As outlined by the petitioner, the source material gum rosin contains a “neutral fraction” (“non-acidic fraction”) besides the resin acids being esterified in GEGR production. This “non-acidic fraction”, which is considered to be at least partly retained during GEGR production and is presumed to result in fraction (c) defined above, is composed of natural esters of resin acids, esters of fatty acids, and unsaponifiables. According to the petitioner, resin acid esters and esters of fatty acids make up the majority of the “non-acidic fraction” (i.e., approximately 60%) in gum rosin. The composition of the resin acid portion of the esters is similar to that of the acid fraction. The fatty acid portion is predominantly composed of C-18 or higher straight-chain acids of various degrees of unsaturation. Soltes and Zinkel describe that most of the non-acidic components of rosins that originate from oleoresin (as gum rosin) are terpenic (e.g. monoterpenes; diterpenes including hydrocarbons, aldehydes, alcohols, methylesters and other esters) (Soltes and Zinkel, 1989; Conner, 1989). From published literature it is well known that the non-acidic fraction of the source material gum rosin is a complex mixture varying with the botanical origin (e.g. Pinus species) and the production process, and comprising as unsaponifiables more than 40 components, e.g. volatile monoterpenes (e.g. alphaterpineol, chavicol methyl ether), diterpene alcohols (e.g. pimarol) and diterpene aldehydes (e.g. pimaral, isopimaral, abietal), the latter two groups being described as “non volatiles” and structurally related to the resin acids (Soltes and Zinkel, 1989; Conner, 1989; Weissmann, 1986). Furthermore, 3,5dimethoxystilbene (pinosylvin dimethyl ether) has been identified in gum rosin as a typical unsaponifiable of Pinus-derived products. According to Conner (1989), various neutral components codistil with the resin acid fraction thus limiting possibilities of purification. The non-acidic fraction of gum rosin includes about 2% of volatile material (Soltes and Zinkel, 1989; Conner, 1989; Joye et al., 1973; Weissmann, 1986). The petitioner states that volatile organic compounds of the non-acidic fraction of gum rosin will be stripped off by the process of counter-current steam distillation in the manufacturing procedure of GEGR. The Panel noted that, according to the description of the petitioner, the gum rosin used as source in the production of GEGR meets the definition of the wood rosin as source of GEWR laid down in the EFSA Journal 2010; 8(7):1654 10 The use of glycerol esters of gum rosin as a food additive Commission Directive 2008/84/EC7 as far as the content of approximately 10% neutrals in the refined gum rosin is concerned. The Panel also noted that a high content of fraction (c) in GEGR of up to 20% results after the esterification and final purification. This implies that the percentage of the unknown substances of fraction (c) is twice the percentage of neutrals in the refined source material. This is unexpected, because the free resin acids in gum rosin have a lower molecular weight than the glycerol esters of the resin acids. Thus the percentage of "other substances" in GEGR should be smaller than the percentage of neutrals/other substances in gum rosin. The higher percentage of other substances in GEGR can also not be due to residues of remaining surplus glycerol, since unreacted glycerol is washed out after esterification. Upon request of the Panel concerning the unexpected high concentration of fraction (c) in GEGR, the petitioner outlined that this is because the GEGR are “manufactured by esterification of refined gum rosin with glycerol at a temperature of as high as 280°C. Esterification at high temperature leads to the formation of unsaponifiables such as colour resulting from the inadvertent oxygen leaking into the reactor (Soltes and Zinkel, 1989). Therefore, the total unsaponifiables in GEGR, newly formed unsaponifiables during esterification plus those originally present in the refined gum rosin, amounts to approximately 20%.” The Panel noted that the composition of fraction (c) is unknown. The possible occurrence of “non volatile” diterpenic substances (e.g. alcohols, aldehydes, esters) in larger amounts and 3,5dimethoxystilbene in fraction (c) has to be taken into consideration. The Panel also noted that the information on the chemical composition of GEGR as proposed by the petitioner is not in line with what would be expected for a botanical preparation (EFSA, 2009). 2.2. Specifications According to the petitioner, GEGR are identified by being insoluble in water and soluble in acetone and toluene, and by comparing the infrared (IR) spectrum of a melted sample on a potassium bromide plate against the standard provided in the Food Chemicals Codex 6th ed. (2008). The physical and chemical parameters, which are proposed by the petitioner for the identification of GEGR and for the determination of the overall purity, are equivalent to those defined by the Commission Directive 2008/84/EC for GEWR. These requirements fulfil those given in the Food Chemicals Codex 6th ed. (2008) and in the new tentative specifications prepared at the 71st JECFA meeting (2009), except for the lead contamination which is limited to not more than 1 mg/kg in both references (Table 1). Table 1: Specifications for GEGR as proposed by the petitioner and as given tentatively by JECFA (JECFA, 2009) Proposal by the petitioner JECFA, 2009 Characteristic of the compound Characteristic of the compound Solubility in water Solubility in acetone insoluble soluble insoluble soluble Sulphur test (Test for absence of tall oil rosin) negative negative > 0.935 (50% solution in d-limonene) > 0.935 (50% solution in d-limonene) Infrared absorption spectrum Specific gravity of the 20 solution [d ] 25 7 Commission Directive 2008/84/EC of 27 August 2008 laying down specific purity criteria on food additives other than colours and sweeteners EFSA Journal 2010; 8(7):1654 11 The use of glycerol esters of gum rosin as a food additive Ring and ball softening point (ºC) 82-90 > 82 3-9 3-9 Hydroxyl Value : 15-45 - Arsenic (mg/kg) <3 - Lead (mg/kg) <2 <1 Mercury (mg/kg) <1 - Cadmium (mg/kg) <1 - Heavy metals (as Pb) (mg/kg) < 10 - 8 Acid Value 9 The JECFA specifications for GEGR (JECFA, 2009) were made tentative pending the submission of IR spectra that correspond to the commercially available products, data on the resin acid composition obtained with updated chromatographic techniques, and additional information on methods that enable the identification of the individual glycerol esters of rosins and their differentiation. This information should be submitted by the end of 2010. The petitioner does not expect the product to contain microorganisms, but data were not given and specifications were not proposed. According to the petitioner, the presence of acrolein and other volatile aldehydes in the final GEGR product is highly unlikely because, at the end of the production process, the product is subjected to steam-stripping followed by 10 micron membrane filtration. The Panel considered whether data on pesticide contamination of GEGR may be relevant for the specifications, since e.g. the use of paraquat is described to intensify the gum exudation from Pinus (Hausen et al., 1982; Hager, 2006). According to the petitioner, the Pinus species used as origin in the production of GEGR is not exposed to any pesticides or additives to intensify the exudation of rosin and therefore pesticide screening of the final GEGR product has not been conducted. The Panel noted that setting limits for glycerol monoesters of resin acids might also be relevant for the specifications. Only the glycerol monoesters of rosins are supposed to undergo partial hydrolysis in the gastrointestinal tract (JECFA, 1996b). The Panel noted that the concentration of free resin acids in GEGR is limited by the acid value which should not exceed 9. The Panel also noted that information on the levels of fraction (b) and fraction (c) is relevant for the specifications. 2.3. Manufacturing process Gum rosin is obtained by collecting the gum (oleoresin) from living pine trees into plastic or clay containers that are then transported via tank trucks for processing. The extract, which is a mixture of rosin (synonym: colophony) and the volatile turpentine oil, is refined by filtration, washing, and distillation to separate these two components. After adding water to the oleoresin, the mixture is heated to 90ºC in order to remove the unusable portion. The mixture is then washed with water and filtered with a metal filter. Additional water is added to the filtered oleoresin, which undergoes a second washing with water. At this step, the mixture is analysed for iron content. The washed oleoresin is filtered again, and then undergoes a direct steam 8 “Acid Value” is defined as the number of mg of potassium hydroxide required to neutralize the acids in 1 g of fatty material (JECFA, 2006). 9 Hydroxyl Value” is defined as the number of mg of potassium hydroxide required to neutralize the amount of acetic acid capable of combining by acetylation with 1 g of sample (JECFA, 2006). EFSA Journal 2010; 8(7):1654 12 The use of glycerol esters of gum rosin as a food additive treatment to separate the turpentine oil and gum rosin components. The gum rosin is analysed for acid number and colour. The gum rosin then undergoes counter-current steam distillation that further separates the material into light material, heavy weight material, and distilled gum rosin. The distilled gum rosin, which is the intermediate product, is tested for colour and acid number. Percent pitch, lignin, and distilled rosin are also monitored to ensure that they are within acceptable quality control limits. The distilled gum rosin is pumped into a batch-type reactor, and esterified with food-grade glycerol under a nitrogen atmosphere. Upon request of the Panel, the petitioner indicated that the chemical reaction of the gum rosin and glycerol is conducted at 260 - 280°C and is allowed to proceed until the desired product specifications are met. These specifications include acid number, colour, and softening point (Ring & Ball Test). This rosin ester is then purified with direct counter-current steam (260°C, 2 hours) and analysed for acid number, colour, and softening point, and is then deodorised. After cooling, the rosin ester is subjected to filtration over a 10-micrometer filter. The filtered product is analysed for acid number, colour, softening point, odour, and taste. The carboxylic group of the resin acids is attached to a tertiary carbon which is sterically hindered. In order to esterify this type of hindered carboxylic groups, generally higher temperatures and more drastic conditions have to be used than for other carboxylic acids. These steric effects are also responsible for the resistance of the resin acid ester linkage to cleavage by water, acid, and alkali (Hausen et al., 1982). It has to be taken into consideration that differences in temperature during esterification may result in differences in degrees of esterification, in stability of formed esters, and in residue concentrations of free acids, which may be relevant for toxicokinetic behaviour and induction of toxicological effects by GEGR. 2.4. Methods of analysis in food According to the petitioner, a method for the quantitative determination of GEGR in beverages is not available. The petitioner referred to a statement of the US Food and Drug Administration (FDA), Division of Food, that the proposed use of the additive GEWR in citrus beverages to adjust the densities is “selflimiting” and therefore the FDA did not require a method of analysis in food. Similarly, neither Directives 95/2/EC, 96/77/EC nor the SCF’s opinions published in 1992 and 1994 have specified an analytical method for determining the quantity of GEWR in beverages as part of its approval of this substance as direct food additive (SCF, 1992; SCF, 1994). A literature research only revealed a paper chromatographic method for the detection of GEWR in ready-to-serve beverages and their concentrates (Nasirullah et al., 1995). 2.5. Stability, reaction and fate in food According to the petitioner, at a temperature of not more than 30ºC, the shelf-life of packed GEGR is six months, although the manufacturer recommends that the product should be used within three months following production. The petitioner refers to studies that show that GEWR are stable in the gastrointestinal tract and that only a minor fraction, most likely the monoglycerol ester fraction undergoes partial hydrolysis. Since GEGR are supposed to be chemically equivalent to GEWR, the petitioner expects that GEGR would be chemically stable in citrus beverages as they are much milder environments compared with the gastrointestinal tract. 2.6. Equivalence of GEGR and GEWR Because of the limited toxicity data available for GEGR, the petitioner submits information and analytical data to demonstrate that GEGR are chemically equivalent to GEWR, claiming that the toxicity data for GEWR are directly relevant to GEGR. EFSA Journal 2010; 8(7):1654 13 The use of glycerol esters of gum rosin as a food additive The equivalence of GEGR and GEWR is primarily examined by looking at physical and chemical parameters and by comparing the chemical composition of fraction (a) (total glycerol esters of resin acids), fraction (b) (free resin acids), and fraction (c) (other substances including unsaponifiables) of GEGR and GEWR under qualitative and quantitative aspects. Raw material processing While gum rosin, the process intermediate esterified to produce GEGR, is obtained by tapping oleoresin gum from living pine trees, wood rosin is obtained via a multistage purification and refining process involving solvent extraction of ground aged pine stumps/pine wood chips followed by solvent-solvent refining of the crude rosin. Though the crude rosin production processes are different, wood rosin and gum rosin are of similar resin acid composition, with some variations. Both gum rosin and wood rosin are process intermediates generally containing approximately 90% resin acids and approximately 10% neutral fraction, which then undergo similar esterification processes for the production of GEGR and GEWR, respectively. The petitioner reported that differences in the non-acidic fractions of gum and wood resin are attributable to their source and manufacture. As also reported in published literature, wood rosin contains small quantities of organic materials present in the solvent extract of pine stumps which are incompletely removed in wood rosin refining. Gum rosin contains other terpenes and hydrocarbons of generally high molecular weight and low volatility in the non acidic fraction that are not stripped off from the oleoresin in turpentine oil recovery. In the esters of resin acids and fatty acids the acidic portion is similar in wood rosin and gum rosin, but the alcohol component may be different (Soltes and Zinkel, 1989). Comparative analysis of representative samples of GEGR and GEWR Comparative analyses of six lots of GEGR and five samples of GEWR (used in the manufacture of beverages) were conducted. Physical and chemical properties were measured, and Infrared (IR) Spectroscopy, Gas Chromatography (GC), and Nuclear Magnetic Resonance (NMR) were used to analyse the products. Comparative IR, GC, and NMR traces of GEGR and GEWR were provided by the petitioner. Physical and chemical property data are presented in Table 2. Table 2: Comparison of physical and chemical properties of GEGR and GEWR, as presented by the petitioner, including concentrations of fractions (a), (b) and (c) Glycerol esters of gum rosin Lot 1190 Lot 1191 Lot 1192 Lot 1193 Lot Lot 1194 1195 Acid Value 6.9 5.9 5.7 5.2 7.1 7.5 Softening Point (ºC) 1 91 92 92 93 91 91 Glycerol esters of wood rosin Mean SD Sample Sample Sample Sample Sample 1 2 3 4 5 Mean SD 5.5 2.8 Physical property 6.4 0.91 2.6 9.7 6.4 3.6 5.3 91.7 0.82 91.5 92 95 91 91 92.1 1.7 Composition (% of product) 2 Fractions a & b (Glycerol Esters of Resin Acids & Free Resin Acids) 84.4 84.2 83.8 84.1 83.5 83.3 83.9 NA 87.2 87.5 87 87.5 87.1 87.3 NA Fraction c3 (Unsaponifiables) 15.6 15.8 16.2 15.9 16.5 16.7 16.1 0.43 12.8 12.5 13 12.5 12.9 12.7 0.23 Ash 0.003 0.003 0.002 0.002 0.003 0.002 0.002 0.001 0.004 0.003 0.004 0.003 0.004 0.004 0.0002 Total 100 100 100 100 100 100 100 100 NA 100 100 100 100 NA 100 Notes: NA: Not applicable; 1 Drop softening point method; 2 Values determined from total of individual resin acids after saponification; 3 Taking into account the results of different analyses showing some batch-to-batch variations for GEGR, the values for fraction (c) range from 15.6 to 20.1% for GEGR; See Table 3 for percentages of individual resin acids after saponification. EFSA Journal 2010; 8(7):1654 14 The use of glycerol esters of gum rosin as a food additive These data demonstrate that the tested physical and chemical properties of these two products are similar. The concentrations of free acids, as measured by the acid value, comprise free resin acids [fraction (b)], and when present other acids (e.g. fatty acids), and are similar for GEGR and GEWR. Some differences are observed for the percentages of the total of fractions (a) and (b) and the percentage of “unsaponifiables” better described as fraction (c) (other substances) of GEGR and GEWR. The values for the total of fractions (a) and (b) range from 83.3 to 84.4% for GEGR and from 87 to 87.5% for GEWR, and for fraction (c) from 15.6 to 16.7% for GEGR and 12.5 to 13% for GEWR. Taking also into account the results of the analysis data for GEGR presented in section 1, the combined values for the total of fractions (a) and (b) range from 79.9 to 84.4%, and for fraction (c) from 15.6 to 20.1% regarding GEGR. The results also show that there are some batch–to-batch variations within GEGR samples. The percentages of individual resin acids measured by GC analysis after saponification of GEGR or GEWR are also in the same range and cover free acids [e.g. fraction (b)], resin acids derived from glycerol esters [fraction (a)], and acids derived from esters of fraction (c) (Table 3). But as mentioned in an FDA response to a petition for the use of GEGR as food additive, this technique may be questionable because it could induce isomerisation of the resin acids, thereby changing the composition as compared to the starting material. No literature references or data were provided to support this statement (Federal Register, 2005). Comparison of the percentages of individual resin acids of total resin acids in glycerol esters of gum rosin and of wood rosin measured by GC analysis after saponification, as presented by the petitioner Table 3: Percent of individual resin acids of total resin acids in glycerol esters of gum rosin Resin Acids Lot Lot Lot Lot 1190 1191 1192 1193 Lot 1195 Mean ±SD Dihydrodextr 0.073 0.063 0.059 0.065 0.079 0.053 opimaric 0.065 0.010 0.777 Tetrahydropi 0.169 0.159 0.157 0.161 0.178 0.164 maric 0.165 0.008 Pimaric Lot 1194 Percent of individual resin acids of total resin acids in glycerol esters of wood rosin Sample Sample 1 2 Sample 3 Sample Sample Mean 4 5 ±SD 0.118 0.253 0.522 0.342 0.402 0.256 0.558 0.218 0.483 0.785 0.771 0.563 0.233 7.64 8.50 7.95 7.78 8.19 7.91 7.99 0.308 5.17 5.63 5.31 6.25 6.45 5.76 0.566 Sandaracopi 7.53 7.33 7.20 maric 7.20 6.74 7.37 7.23 0.267 5.76 5.82 6.05 6.41 6.40 6.09 0.308 Communic 9.91 10.51 9.92 10.0 0.253 10.7 10.1 10.6 10.3 9.69 10.3 0.405 10.03 10.11 9.79 Palustric 4.99 5.74 5.65 5.88 6.03 5.92 5.70 0.373 5.59 4.68 5.27 4.29 5.90 5.14 0.659 Isopimaric 9.37 8.29 8.53 8.47 8.02 8.63 8.55 0.453 15.0 12.7 12.1 11.9 9.35 12.2 2.01 Abietic 25.3 25.6 24.1 24.0 23.2 24.5 24.5 0.867 19.5 22.0 24.1 24.5 26.0 23.2 2.53 Dehydroabiet 23.7 24.3 24.2 ic 24.5 23.8 23.0 23.9 0.548 34.6 28.1 25.4 24.5 22.7 27.1 4.62 Neoabietic 1.61 1.75 2.08 2.14 1.83 1.68 1.85 0.217 0.546 2.28 2.20 1.66 2.77 1.89 0.848 Other unspecified acids 9.67 8.13 10.27 9.86 11.3 10.9 10.0 1.12 1.87 8.33 8.26 8.82 9.61 7.38 3.13 100 100 100 100 100 NA 100 NA Total1 100 100 100 100 100 100 100 Notes: 1 The total of 100% refers to the resin acids obtained after saponification. Thus the percentages given do not represent the portions of the resin acids moiety present in GEGR. Five separate studies compared two batches of GEGR and five batches of GEWR. Analyses included Fourier Transform Infrared Spectroscopy (FTIR) to investigate sample compositions, Differential Scanning Calorimetry (DSC) to investigate the thermal behaviours, NMR Spectroscopy to determine the chemical structures, image analysis using optical microscopy to determine softening and melting EFSA Journal 2010; 8(7):1654 15 The use of glycerol esters of gum rosin as a food additive temperatures and Gas Chromatography-Mass Spectrometry (GC/MS) with head space sampling to analyse Volatile Organic Compounds (VOCs). IR spectra of two GEGR and four GEWR samples showed similar ester and hydroxyl signals although varying in intensity. GEWR showed a higher degree of variability than GEGR. Similar proton NMR spectra were obtained, although small differences were indicative of the different degrees of esterification. DSC thermograms indicated that both GEGR and GEWR have similar thermal behaviours, and that they are both practically amorphous. Headspace GC/MS indicated only small differences in some volatiles present at low concentrations, and confirmed that GEWR had a higher total content of volatile organic compounds which agrees with the result from the gravimetric analysis of VOCs showing 0.99% VOCs in the GEWR sample and 0.1% VOCs in the GEGR sample. Variability in softening and melting temperatures was found for both GEWR and GEGR possibly due to variability in the proportions of the resin acids besides differences in degree of esterification, content of VOCs and other trace compounds. The results also confirmed other results indicating that GEWR have a higher degree of variability than GEGR. According to the petitioner, currently there are no data available concerning the identification or quantification of individual components in fractions (a), (b), and (c) of GEGR and GEWR as a basis for comparison. The Panel noted that the concentrations of individual resin acids measured in GEWR and GEGR samples after saponification, covering the fraction of free resin acids [fraction (b)], the resin acids derived from glycerol esters [fraction (a)], and the acids derived from esters of fraction (c), are very similar. But apart from the missing qualitative and quantitative analysis of individual glycerol mono- diand triesters in fraction (a) of GEGR and GEWR, most notably the components of their fraction (c), which may differ due to different manufacturing procedures and to different botanical sources, are unknown. Since the content of fraction (c) in GEGR accounts for up to 20%, this information is most relevant for the evaluation of the equivalence of GEGR and GEWR. Furthermore, the Panel noted that the occurrence of a residual fraction of monoglycerol esters [in fraction (a)] which is a component of GEGR is not mentioned in the definition of GEWR according to Commission Directive 2008/84/EC and to the actual JECFA specifications (JECFA, 2009), in which GEWR are described as a complex mixture of tri- and diglycerol esters of resin acids from wood rosin. The Panel could not conclude based on the chemical data provided, that GEGR and GEWR are chemically equivalent. 2.7. Case of need and proposed uses GEGR are intended for use as weighting agents in oil-based citrus flavourings for beverages. Being typically lipophilic components, their function is to increase the density of citrus oils (e.g., lemon oil, orange oil). According to the petitioner, this results in improved cloud stability when they are dispersed in the finished beverage at a maximum concentration of 100 mg/l. These beverages are oil-in-water emulsions having typically an opaque or cloudy appearance. Beverage emulsions are thermodynamically unstable and have a tendency to separate into two immiscible liquids. One of the approaches to control beverage emulsion stability is to minimise the density contrast between the oil phase and the aqueous phase with the use of weighting agents. 2.8. Information on existing authorisations and evaluations Current regulatory status of rosin glycerol esters in the EU and evaluations by JECFA and the Codex Committee on Food Additives (CCFA) Directive 95/2/EC allows the use of GEWR (E 445) in non-alcoholic flavoured cloudy drinks and certain cloudy spirit drinks up to a maximum level of 100 mg/l and for the surface treatment of citrus fruit up to a maximum level of 50 mg/l. However, the specification of purity for GEWR established by EFSA Journal 2010; 8(7):1654 16 The use of glycerol esters of gum rosin as a food additive Commission Directive 2008/84/EC presently excludes esters derived from gum rosin, an exudate of living pine trees, and substances derived from tall oil rosin, a by-product of Kraft (paper) pulp processing. The safety in use of GEWR (E 445) has been evaluated by the SCF in 1992 which allocated an Acceptable Daily Intake (ADI) of 12.5 mg/kg bw/day. This ADI was derived from the results of a 90day rat study which indicated a No-Observed-Adverse-Effect Level (NOAEL) of 2500 mg/kg bw/day applying a safety factor of 200 to take into account the 90-day duration (SCF, 1994). GEGR have been evaluated by JECFA in its 71st meeting in 2009 upon request from the same petitioner providing the data for the present evaluation (JECFA, 2010). The JECFA decided to include GEGR in the ADI for GEWR of 0 – 25 mg/kg bw/day, thereby establishing a group ADI of 0 – 25 mg/kg bw/day for GEWR and GEGR. The specifications for GEGR were made tentative pending the submission of IR spectra that correspond to the commercially available products, data on the resin acid composition obtained with updated chromatographic techniques, and additional information on methods that enable the identification of the individual glycerol esters of rosins and their differentiation. This information should be submitted by the end of 2010. Furthermore, the Committee requested that it be provided with full reports of the two 90-day toxicity studies in rats fed dietary concentrations of up to 1.0 % GEGR, to confirm the validity of the comparison of GEWR with GEGR (JECFA, 2009 and 2010). The new tentative specifications for GEGR have been published as have been those for GEWR and GETOR (JECFA, 2009). GETOR have been evaluated by JECFA in its 71st meeting in 2009 (JECFA, 2010). The Committee concluded in principle that the data from GEWR could be used in the evaluation of GETOR; however, the Committee did not have adequate information on the composition of GETOR, considering that the source material and production processes are different, which may result in different by-products. The Committee decided that it could not evaluate GETOR without additional information on its composition in order to clarify the extent and significance of any differences relative to other glycerol esters of rosins. The specifications for GETOR were made tentative pending the submission of infrared spectra that correspond to the commercially available products, data on the resin acid composition obtained with updated chromatographic techniques, and additional information on methods that enable the identification of the individual glycerol esters of rosins and their differentiation. The Committee also requested information on the identity of the sulphur compounds in the commercial product. This information should be submitted by the end of 2010 (JECFA, 2009). No ADI was allocated for GETOR at the 71st meeting of JECFA (JECFA, 2009 and 2010). GEWR were evaluated by JECFA in 1996. Although there were no long-term studies of toxicity or reproductive toxicity available, the Committee considered that the data from previously reviewed studies and the new studies confirming the non-bioavailability of GEWR were adequate to establish an ADI. Therefore, on the basis of the 13-week toxicity study in rats with food-grade material, in which the NOAEL was 2500 mg/kg bw/day, by applying a safety factor of 100, the Committee allocated an ADI of 0 - 25 mg/kg bw/day, in which recently GEGR was included as mentioned above. JECFA established in its 71st meeting in 2009 new specifications for GEWR, which were made tentative pending the submission of infrared spectra that correspond to the commercially available products, data on the resin acid composition obtained with updated chromatographic techniques, and additional information on methods that enables the identification of the individual glycerol esters of rosins and their differentiation. This information should be submitted by the end of 2010 (JECFA, 1996b; JECFA, 1997; JECFA, 2009). The Panel noted the recent development at the 42nd session of the Codex Committee on Food Additives (CCFA) on 15 -19 March 2010 regarding the revision of the International Numbering System (INS) for the existing INS number 445 (“glycerol ester of wood rosin”). The Panel noted that the Committee recommended allocating the following INS numbers to “glycerol ester of gum rosin” (GEGR) (INS 445 (i)), “glycerol ester of wood rosin” (GEWR) (INS 445 (iii)) and “glycerol ester of tall oil rosin” EFSA Journal 2010; 8(7):1654 17 The use of glycerol esters of gum rosin as a food additive (GETOR) (INS 445(ii)), all falling down under the newly renamed INS 445 "glycerol esters of rosin", referring to similarities of these food additives. Current FDA regulatory status of rosin glycerol esters In the USA, GEGR, as well as GEWR and GETOR, are approved for use in chewing gum bases (21 CFR §172.615), as a food additive to adjust the density of citrus oils used in the preparation of beverages, provided that the amount of the additive does not exceed 100 mg/l of the finished beverage (21 CFR §172.735), and for several indirect additive uses (manufacture of articles or components of articles intended for use in producing, manufacturing, packing, processing, preparing, treating, packaging, transporting, or holding food) (21 CFR §178.3870, Federal Register, 2007). The US FDA concluded in its evaluation of GEGR for the above mentioned uses as a food additive: “Because the agency has determined that GEGR and GEWR are similar with respect to the identity of their chemical components and that any difference in the ranges for the components of GEGR and GEWR are not significantly different and would be of no toxicological concern, there is no need for toxicological testing of GEGR to demonstrate that the petitioned use is safe” (Federal Register, 2005). 2.9. Exposure The petitioner estimated consumption of GEGR in citrus beverages based on data contained in the 94-96 Continuing Surveys of Food Intakes by Individuals (94-96 CSFII) (Agricultural Research Service, 1998). The 94-96 CSFII is a dataset from national food consumption surveys conducted by the US Department of Agriculture (USDA). The 94-96 CSFII was a three-year survey in which data were collected from a stratified area probability sample of individuals residing in households in the US. Households represented a cross-section of the population of the 50 states and the District of Columbia. Intake estimates for GEGR The petitioner estimated the projected intake of GEGR by assuming that this material was present in all citrus beverages at a concentration of 100 mg/l. Based on the estimates provided by the petitioner, the exposure to GEGR from citrus beverages of the US population is 2.5 mg/day (0.04 mg/kg bw/day on a 60 kg bw basis) on average. If calculating the exposure to GEGR only for those individuals actually consuming these beverages, the petitioner reported this to be 43.3 mg/day on average (0.72 mg/kg bw/day) and 75.0 mg/day at the 90th percentile (1.25 mg/kg bw/day) The Panel calculated the exposure to GEGR based on the information available from the EFSA Concise Database in Exposure Assessment on the intake of soft drinks with percentage of fruits lower than nectars (food category 7B) and based on the intended level of use in those beverages of 100 mg/l. The exposure to GEGR from its use in spirit drinks was regarded by the Panel to be negligible. The average exposure of the total population to GEGR would range across Europe from 4.2 to 33 mg/day (0.07 to 0.74 mg/kg bw/day) and from 29 to 200 mg/day at the 97.5th percentile (0.5 to 3.3 mg/kg bw/day). Since the EFSA Concise Database in Exposure Assessment does not provide data on the intake of nonalcoholic flavoured drinks by children, the Panel also calculated the exposure to GEGR based on the UK data for children using the intake of non-alcoholic flavoured drinks and the intended use level of 100 mg/l. The exposure was estimated to be 1.7 mg/kg bw/day on average and 5.8 mg/kg bw/day at the 97.5th percentile. The Panel noted that these estimates are conservative, since GEGR is not intended to be added to all non-alcoholic flavoured drinks but to citrus-fruit based drinks only. 3. Biological and toxicological data According to the petitioner, the submitted toxicity data for GEWR are directly relevant to GEGR because it is assumed that GEGR and GEWR are essentially equivalent. The Panel noted that, based on the chemical data provided, it could not be concluded that GEGR and GEWR are chemically equivalent. Therefore the Panel could not base the safety evaluation of GEGR on the results of toxicological studies EFSA Journal 2010; 8(7):1654 18 The use of glycerol esters of gum rosin as a food additive available for GEWR (Blair, 1991, 1992, and 1995; Noker, 1996; Lin, 1996; Kay, 1960c; Ishidate et al., 1984; Murli, 1988; Cifone, 1988; Mukherjee et al., 1992). The petitioner submits supplemental subchronic and 2-year oral toxicity studies that have been performed on the parent materials, gum rosin and wood rosin, respectively, which have been performed in the 1960s as part of a comprehensive testing program to evaluate the toxicity of different rosin derivatives (Kay 1960a,b; Kohn, 1962a-d). According to the petitioner, these studies cannot be relied upon as the primary basis for the safety evaluation of GEGR because it could not be established that these studies had undergone independent validation and some of them have been disqualified by the FDA as a result of an investigation that revealed improper practices in the conducting laboratory. The Panel noted that these studies may not be relevant for the safety evaluation of GEGR and GEWR. Due to the harsh manufacturing conditions of glycerol esters of rosins, including heating at high temperatures e.g. isomerisation, oxidation and dehydrogenation-hydrogenation reactions of the resin acids (whether as individual free acids or bound in esters) may occur. Decarboxylation of resin acids may also take place. In consequence, the composition of resin acids in the glycerol esters of rosins is expected to differ from that of the original rosin (Soltes and Zinkel, 1989; Downs and Sansom, 1999). 3.1. Absorption, distribution, metabolism and excretion 3.1.1. Glycerol esters of gum rosin No data available. 3.1.2. Free resin acids - Fraction (b) JECFA reviewed the results of an unpublished kinetic study on the absorption and elimination of several free resin acids submitted in support of the JECFA food additive evaluation of GEWR (Radomski, 1965; JECFA 1996a). The following is a summary of the report by JECFA. In recovery experiments, 4 male rats received a single oral dose of 100 mg (300 mg/kg bw) or 1 mg (3 mg/kg bw) tritiated resin acids from wood rosin (dehydroabietic, tetrahydroabietic or isopimaric acids) as a 5% solution in corn oil. After administration of dehydroabietic acid, the rats excreted on average 80% of the 100 mg dose in faeces, and 7.2% in urine over a 15-day post-treatment period. Total recoveries in the 4 animals ranged from 71% to 99% at the end of 15 days. Four additional total recovery experiments were conducted, utilizing a 1 mg dose of labelled dehydroabietic acid, and sampling carried out at various times ranging from 28 to 51 hours post-treatment. In these studies, the amount of dehydroabietic acid recovered averaged 70% in faeces, 8% in urine, 17% in the GI tract, 0.5% in breath, and 1% in the carcass, for a total recovery of 96.5%. In recovery experiments with tetrahydroabietic acid, 2 rats were given 45.7 µCi of the tritiated derivative and the radioactivity monitored at regular intervals after administration (1, 2, 3, 4, 5-8, 9-12 and 13-16 days). Recovery of tritiated tetrahydroabietic acid averaged 92% in faeces, 5% in urine and 3% in breath for all average total recovery of 100%. In concurrent studies with isopimaric acid, recovery also averaged 100% total with 83% found in faeces, 15% in urine, and 2% in breath. Four rats were given 30 µCi (105 mg) dehydroabietic acid and quantitative chromatographic analyses were made of faeces and urine collected over 2 days. Analysis revealed 3 major metabolites, which were not identified but for convenience were called A, B, and C. Recovery in faeces amounted to 89% of the administered activity, of which 14% was dehydroabietic acid, 33% metabolite A, 8% metabolite B, and 14% metabolite C. Of the 8% of the administered activity recovered in urine, 0.13% was dehydroabietic acid, 7% was metabolite B, and 0.55% metabolite C. In rats given labelled tetrahydroabietic or isopimaric acids, the bulk of the radioactivity in faeces and urine consisted of the unchanged acids. In tissue distribution studies with dehydroabietic acid, 4 male and 4 female rats each were given 50 mg (5.5 µCi), and distribution of radioactivity measured at various time intervals after administration (1, 2, 4 or 8 hours). Radioactivity was distributed among all major organs, fat, and muscle, with peak levels EFSA Journal 2010; 8(7):1654 19 The use of glycerol esters of gum rosin as a food additive occurring 4 hours after administration. After 8 hours, the highest concentrations of radioactivity were found in the liver (approximately 12%) and in kidney (approximately 5%) (Radomski, 1965; JECFA 1996a). 3.2. Toxicological data 3.2.1. Acute oral toxicity No data available for GEGR. 3.2.2. Short-term and subchronic toxicity In a 1996 “white paper”, the National Association of Chewing Gum Manufacturers and European Association of the Chewing Gum Industry (NACGM/EACGI) summarised the results of two 90-day toxicity studies on GEGR from which more detailed data cannot be made available to the Panel because they are confidential business information. The study results as reported by the NACGM/EACGI (1996) are as follows: “Two subchronic toxicity studies were conducted in 1989 in which rats were fed GEGR at dietary levels of 0.2, 0.5 and 1% for 90 days. The no-effect level in each study was determined to be 1% in the diet” (NACGM/EACGI, 1996). Based on standard conversion methods a body weight of 400 g/animal and a food consumption of 20 g/day are assumed (IPCS, 1987; IPCS, 1990). Thus a dietary level of 1% is equivalent to an intake of 500 mg/kg bw/day. 3.2.3. Genotoxicity 3.2.3.1. Glycerol esters of gum rosin No data available. 3.2.3.2. Free resin acids - Fraction (b) In the Salmonella/mammalian-microsome assay, neoabietic acid showed dose-related increases in mutagenicity in TA1535, TA100, TA1538, and TA98 strains but not in the TA1537 strain. Metabolic activation with a preparation of Aroclor 1254-induced liver homogenate slightly reduced the mutagenic responses. Negative responses were found for abietic acid, dehydroabietic acid, pimaric acid, isopimaric acid, and sandaracopimaric acid (Nestmann et al., 1979; JECFA, 1996a). Mutagenicity testing of neoabietic acid in growing yeast cells (strain XV185-14C) without S-9 fraction gave a positive response (Nestmann et al., 1983; JECFA, 1996a). The Panel noted that there are no data from in vivo genotoxicity testing that could overrule these positive in vitro findings. 3.2.4. Chronic toxicity and carcinogenicity No data are available for GEGR. 3.2.5. Reproductive and developmental toxicity No data are available for GEGR. 3.2.6. Other studies/Contact sensitisation Since 1980, the number of reported cases of contact allergy to rosin has increased and rosin has become one of the most important dermal sensitisers. Dermal contact sensitisation is seen with different types of rosins and modified rosins and their components as well as with GEGR, GEWR, and GETOR (e.g. Downs and Sansom, 1999, Gaefvert et al., 1994, Hausen and Mohnert, 1989; Shao et al., 1993; Geier and Hausen, 2006; Illing et al., 2009). EFSA Journal 2010; 8(7):1654 20 The use of glycerol esters of gum rosin as a food additive Even though it was not possible to sensitise guinea pigs against purified abietic acid (Karlberg et al., 1985), Hausen and Mohnert (1989) found that of 44 dermatologic patients, 17 (39%) reacted positive to purified abietic acid as well as to standard rosin in a patch test. As reviewed by Karlberg et al. (1999) and Downs and Sansom (1999) abietic type acids are easily oxidised when in contact with air, and the oxidation products are thought to be contact sensitisers. Apart from resin acids and esters of resin acids, the non-acidic part of a rosin has provoked positive reactions in human patch testing (Hausen and Mohnert, 1989). The allergenicity of GEGR and their components has been investigated in guinea pigs and in human patch tests. In studies in guinea pigs 1-glycerol monoabietate was found to be a sensitiser, while glycerol triabietate was not. No significant cross-reactivity to these compounds was found in the animals sensitive to unmodified gum rosin. Of 12 patients sensitive to gum rosin, five showed a concomitant reaction to 1-glycerol monoabietate. The majority of the patients reacted positive in patch tests with GEGR (containing 0.3% abietic acid and 2.8% 1-glycerol monoabietate). No reactions were seen in rosin-sensitive patients tested with 1,2-glycerol diabietate, 1,3-glycerol diabietate or glycerol triabietate. According to the authors, the difference in contact sensitisation potential of 1-glycerol monoabietate and glycerol triabietate may be due to their difference in size. 1-glycerol monoabietate having only about 1/3 of the molecular weight of glycerol triabietate would probably have a higher bioavailability across the skin than the latter. The authors consider 1,2-glycerol diabietate and 1,3glycerol diabietate to be of limited clinical significance (Gaefvert et al., 1994; Shao et al., 1993). A case of an 8-year old boy who suffered from recurring perioral dermatitis for 18 months was reported. He frequently chewed gum before each episode of dermatitis. Patch testing was positive for cobalt, rosin, fragrance-mix, oakmoss and isoeugenol as well as to the previously used chewing gum and bubble gum. The perioral dermatitis improved but did not completely disappear after the child stopped chewing gum. The authors assumed that the perioral dermatitis was due to rosin in chewing gum, but possible sensitivity to allergens other than rosin could not be ruled out (Satyawan et al., 1990). Two additional cases of adverse reactions in the mouth following chewing gum exposure are reported, in which patients showed positive results of patch testing with rosin (Gupta and Forsyth, 1999). The Panel noted that there is no evidence of a causal relationship in these 3 cases between the adverse effects observed and exposure to chewing gum which possibly might have contained rosin or rosinderived products. The question arises whether the oral exposure with the low concentrations of GEGR used in beverages could induce systemic contact dermatitis or elicit flare-up reactions in individuals with a known contact hypersensitivity to rosin or modified rosin products. From challenge studies it is known that in e.g. nickel-sensitive, poison ivy-sensitive, clonidine-sensitive, and balsam of Peru-sensitive patients with different oral doses of the appropriate contact allergens flares at the site of the original dermatitis in 1% to 10% of the patients were induced. In some cases, a marked dose-response relationship was observed. In other cases, such correlations did not occur, but even placebos were capable of eliciting a flare-up reaction (Hausen, 1998). Literature searches did not reveal reports of allergic responses from ingestion of GEGR in beverages. 4. Discussion The present opinion assesses the use of GEGR as a stabiliser and emulsifier in certain non alcoholic flavoured cloudy drinks and certain cloudy spirit drinks to a maximum level of 100 mg/l. GEGR have been evaluated by JECFA in its 71st meeting in 2009. The Committee established a group ADI of 0 – 25 mg/kg bw/day for GEWR and GEGR. The specifications for GEGR were made tentative pending the submission of IR spectra that correspond to the commercially available products, data on the resin acid composition obtained with updated chromatographic techniques, and additional information on methods that enable the identification of the individual glycerol esters of rosins and their EFSA Journal 2010; 8(7):1654 21 The use of glycerol esters of gum rosin as a food additive differentiation. This information should be submitted by the end of 2010. Furthermore, the Committee requested that it be provided with full reports of the two 90-day toxicity studies in rats fed dietary concentrations of up to 1.0 % GEGR, to confirm the validity of the comparison of GEWR with GEGR. GEGR are obtained by esterification of refined gum rosin originating from Pinus oocarpa Schiede. GEGR are described by the petitioner as a complex mixture of tri- and diglycerol esters of resin acids from gum rosin, with glycerol triabietate as the main component and a residual fraction of glycerol monoesters. Besides the glycerol esters of resin acids [fraction (a)], which amount only to 75.7 - 78.2% of GEGR, 2.8 - 4.2% free resin acids [fraction (b)], and 19 - 20.1% other non acidic saponifiable and unsaponifiable substances [fraction (c)] are present in GEGR as impurities according to the analysis data of three samples provided by the petitioner upon request from the Panel. The petitioner provided data on the percentages of resin acids derived from fractions (a), (b) and (c) after saponification of GEGR. Abietic acid (24.5%), dehydroabietic acid (23.9% ) and communic acid (10.0%) were the main components (mean) besides isopimaric acid (8.6%), pimaric acid (8.0%), sandaracopimaric acid (7.2%), palustric acid (5.7%), neoabietic acid (1.9%), tetrahydropimaric acid (0.17%), dihydrodextropimaric acid (0.07%), and some unspecified acids (10.0%). The percentages given refer to the total of 100% of resin acids obtained after saponification and do not represent the portion of the resin acids moiety present in GEGR. The Panel noted that the petitioner did not provide data on the identity and quantity of individual components in fractions (a), (b) and (c) of unsaponified GEGR. The Panel also noted that the proportions of glycerol monoesters, (1,2)-glycerol diesters, (1,3)-glycerol diesters and glycerol triesters in fraction (a) are unknown. The petitioner gave some information concerning the composition of the non-acidic fraction of the source material gum rosin, which is presumed to be at least partly retained during GEGR production and to result in fraction (c). According to this information, the non-acidic fraction of gum rosin is composed of natural esters of resin acids, and esters of fatty acids, which together make up the majority of the non acidic fraction (i.e., approximately 60%), and of unsaponifiables. In published literature it is described that most of the non acidic components of rosins that originate from oleoresin (as gum rosin) are terpenic (e.g. monoterpenes, diterpenes including hydrocarbons, aldehydes, alcohols, methylesters and other esters). The unsaponifiables in gum rosin comprise more than 40 components, e.g. volatile monoterpenes (e.g. alpha-terpineol, chavicol methyl ether), diterpene alcohols (e.g. pimarol) and diterpene aldehydes (e.g. pimaral, isopimaral, abietal), the latter two groups being described as “non volatiles” and structurally related to the resin acids. Furthermore, 3,5-dimethoxystilbene (pinosylvin dimethyl ether) has been identified in gum rosin as a typical unsaponifiable of Pinus derived products. The non acidic fraction of gum rosin includes about 2% of volatile material. The petitioner states that volatile organic compounds of the non acidic fraction of gum rosin will be stripped off by the process of counter-current steam distillation in the manufacturing procedure of GEGR. The Panel noted that according to the description of the petitioner, the gum rosin used as source in the production of GEGR meets the definition of the wood rosin as source of GEWR laid down in the Commission Directive 2008/84/EC as far as the content of approximately 10% neutrals in the refined gum rosin is concerned. But the Panel also noted that a high content of fraction (c) in GEGR of up to 20% results after the esterification and final purification. This implies that the percentage of the unknown substances of fraction (c) is twice the percentage of neutrals in the refined source material. According to the petitioner, this increase is due to the new formation of unsaponifiables during the process of esterification at high temperature in the presence of oxygen. The composition of fraction (c) is unknown. The possible occurrence of “non volatile” diterpenic substances (e.g. alcohols, aldehydes, esters) in larger amounts and of 3,5-dimethoxystilbene in fraction (c) has to be taken into consideration. EFSA Journal 2010; 8(7):1654 22 The use of glycerol esters of gum rosin as a food additive The extent to which compounds of different volatility are removed in the course of manufacturing is unknown. The Panel noted that the data provided to chemically characterise GEGR are not sufficient since qualitative and quantitative data on individual components of the three fractions are missing. Especially levels of compounds expected to be bioavailable (glycerol monoesters of resin acids, free resin acids, unsaponifiables) should have been analysed individually. The Panel also noted that the information on the chemical composition for GEGR as proposed by the petitioner is not in line with what would be expected for a botanical preparation (EFSA, 2009). For the specification of GEGR, physical and chemical parameters, which are equivalent to those defined by the Commission Directive 2008/84/EC for GEWR, have been proposed by the petitioner. The concentration of free acids in GEGR is limited by the acid value which should not exceed 9. The Panel noted that setting limits for glycerol monoesters of resin acids, which are supposed to undergo partial hydrolysis in the gastrointestinal tract, and furthermore information on the levels of fraction (b) and fraction (c) might be relevant for the specifications. The Panel noted that for GEGR no studies are available on: i) absorption, distribution, metabolism and excretion, ii) short-term and subchronic toxicity, iii) genotoxicity, iv) chronic toxicity and carcinogenicity, v) reproductive and developmental toxicity. In a “white paper”, the National Association of Chewing Gum Manufacturers and European Association of the Chewing Gum Industry (NACGM/EACGI) summarised the results of two 90-day toxicity studies in which rats were fed GEGR at dietary levels of 0.2, 0.5 and 1% for 90 days. The no-effect level in each study was determined to be 1% in the diet being equivalent to an intake of 500 mg/kg bw/day. Since more detailed data cannot be made available to the Panel because they are confidential business information, these studies cannot be evaluated and taken into account. In the Salmonella/mammalian-microsome assay, neoabietic acid showed dose-related increases in mutagenicity in TA1535, TA100, TA1538, and TA98 strains but not in the TA1537 strain. Negative responses were found for abietic acid, dehydroabietic acid, pimaric acid, isopimaric acid, and sandaracopimaric acid. Mutagenicity testing of neoabietic acid in growing yeast cells (strain XV18514C) without S-9 fraction gave a positive response. The Panel noted that there are no data from in vivo genotoxicity testing that could overrule these positive in vitro findings. Due to the limited toxicity data available for GEGR the petitioner submits analytical data to demonstrate that GEGR are chemically equivalent to GEWR (E 445). On this basis, the petitioner claims that toxicity data for GEWR are directly relevant to GEGR. GEWR have already been authorised by Directive 95/2/EC10, allowing their use as a stabiliser and emulsifier in non-alcoholic flavoured cloudy drinks and certain cloudy spirit drinks up to a maximum level of 100 mg/l. Comparative analyses of GEGR and GEWR samples were conducted. Physical and chemical properties were measured and analyses included Infrared (IR) Spectroscopy, Gas Chromatography (GC), Nuclear Magnetic Resonance (NMR), Differential Scanning Calorimetry (DSC) and Gas Chromatography-Mass Spectrometry (GC/MS). The results demonstrate that the tested physical and chemical properties of these two products are similar. The concentrations of free acids, as measured by the acid value, comprise free resin acids [fraction (b)] and are similar for GEGR and GEWR. The percentages of individual resin acids obtained after saponification of GEGR or GEWR are also in the same range (Table 3) and cover free acids [e.g. fraction (b)], resin acids derived from glycerol esters [fraction (a)], 10 European Parliament and Council Directive 95/2/EC of 20 February 1995, on food additives other than colours and sweeteners EFSA Journal 2010; 8(7):1654 23 The use of glycerol esters of gum rosin as a food additive and acids derived from esters of fraction (c). Taking into account the results of different analyses showing some batch-to-batch variations for GEGR, the values for fraction (c) range from 15.6 to 20.1% for GEGR and 12.5 to 13% for GEWR. According to the petitioner, currently there are no data available concerning the identification or quantification of individual components in fractions (a), (b) and (c) of GEGR and GEWR as a basis for comparison. The petitioner reported that differences in the non-acidic fractions of the source materials gum and wood rosin are attributable to their source and manufacture. While Pinus palustris is indicated in a previous JECFA evaluation as the source for wood rosin used in the production of GEWR (JECFA, 1996b), the species from which gum rosin for GEGR production is obtained is specified by the petitioner to be Pinus oocarpa Schiede. As reported in published literature, wood rosin contains small quantities of organic materials present in the solvent extract of pine stumps which are incompletely removed in wood rosin refining. Gum rosin contains other terpenes and hydrocarbons of generally high molecular weight and low volatility in the non acidic fraction that are not stripped off from the oleoresin in turpentine oil recovery and it is not known to which extent they are removed in the course of manufacturing of refined gum rosin and GEGR production. In the natural esters of resin acids and fatty acids the acidic portion is similar in wood rosin and gum rosin but the alcohol component may be different. The Panel noted that the concentrations of individual resin acids measured in GEWR and GEGR samples after saponification, covering the fraction of free resin acids [fraction (b)], the resin acids derived from glycerol esters [fraction (a)] and the acids derived from esters of fraction (c), are very similar. But apart from the missing qualitative and quantitative analysis of individual glycerol mono- diand triesters in fraction (a) of GEGR and GEWR, most notably the components of fraction (c) of GEGR and GEWR, which may differ due to different manufacturing procedures and to different botanical sources, are unknown. Since the content of fraction (c) in GEGR accounts for up to 20%, this information is most relevant for the evaluation of the equivalence of GEGR and GEWR. The Panel could not conclude based on the chemical data provided, that GEGR and GEWR are chemically equivalent. Therefore, the Panel could not base the safety evaluation of GEGR on the results of toxicological studies available for GEWR. Considering different botanical sources and manufacturing procedures for GEGR and GEWR which may lead to differences in composition, and taking into account that JECFA made the recently published specifications for GEGR and GEWR only tentative, the Panel noted that the chemical equivalence of GEGR and GEWR has to be carefully examined and could only be demonstrated by comparing the proportions of all different fractions in GEGR and GEWR and the quantitative analyses concerning their individual components. Although dermal contact sensitisation is seen with different types of rosins and modified rosins and their components, as well as with GEGR, from ingestion of GEGR in beverages, allergic responses have not been reported. Therefore the Panel did not consider it likely that the oral exposure via the intended use and use levels of GEGR in beverages is associated with a relevant risk of adverse effects for individuals with a known contact hypersensitivity to rosin or modified rosin products. The exposure to GEGR was calculated based on the information available from the EFSA Concise Database in Exposure Assessment on the intake of soft drinks with percentage of fruits lower than nectars (food category 7B) and based on the intended level of use in those beverages of 100 mg/l. The exposure to GEGR from its use in spirit drinks was regarded by the Panel to be negligible. The average exposure of the total population to GEGR would range across Europe from 4.2 to 33 mg/day (0.07 to 0.74 mg/kg bw/day) and from 29 to 200 mg/day at the 97.5th percentile (0.5 to 3.3 mg/kg bw/day). EFSA Journal 2010; 8(7):1654 24 The use of glycerol esters of gum rosin as a food additive Since the EFSA Concise Database in Exposure Assessment does not provide data on the intake of nonalcoholic flavoured drinks by children, the Panel also calculated the exposure to GEGR based on the UK data for children using the intake of non-alcoholic flavoured drinks and the intended use level of 100 mg/l. The exposure was estimated to be 1.7 mg/kg bw/day on average and 5.8 mg/kg bw/day at the 97.5th percentile. The Panel noted that these estimates are conservative, since GEGR is not intended to be added to all non-alcoholic flavoured drinks but to citrus-fruit based drinks only. CONCLUSIONS The present opinion deals with the safety of GEGR when used as a food additive with the function of a stabiliser and emulsifier in certain types of drinks. GEGR are described by the petitioner as a complex mixture of tri- and diglycerol esters of resin acids from gum rosin, with a residual fraction of monoglycerol esters. The Panel concluded that the chemical and toxicological characterisation of GEGR is not adequate and that the absence of toxicological study reports on GEGR prevents the evaluation of the safety of GEGR. The Panel also concluded that there is not sufficient information to evaluate the chemical equivalence of GEGR and GEWR and that therefore the toxicological data obtained with GEWR could not be used for read across to GEGR. The Panel concluded that the available data are too limited to conclude on the safety of GEGR as a food additive at the proposed uses and use levels. EFSA Journal 2010; 8(7):1654 25 The use of glycerol esters of gum rosin as a food additive DOCUMENTATION PROVIDED TO EFSA 1. Food additive petition to amend European Parliament and Council Directive 95/2/EC and Commission Directive 96/77/EC with the addition of glycerol ester of gum rosin by Spherix Incorporated, Bethesda for T & R Chemicals Inc. Clint, Texas, November 2008. REFERENCES Agricultural Research Service (ARS), 1998. Continuing Survey of Food Intakes by Individuals 1994-96. U.S. Department of Agriculture. Computer Tapes. Blair M, 1991. Ester Gum 8BG. 13-week dietary toxicity study in rats. Unpublished Report No. 548007 from International Research and Development Corporation, Mattawan, MI. Submitted to WHO by Hercules Inc., DE. Blair M, 1992. Ester Gum 8BG. 13-week dietary toxicity study in rats. Amendment to the final report. Unpublished Report No. 548-007 from International Research and Development Corporation, Mattawan, MI. Submitted to WHO by Hercules Inc., DE. (Reported in JECFA 1996a). Blair M, 1995. A dietary excretion study with Ester Gum 8BG in Fischer 344 rats. Unpublished Report No. 3352.2 from Springborn Laboratories, Inc., Spencerville, OH. Submitted to WHO by Hercules Inc., DE. Reported in JECFA (1996b). Cifone MA, 1988. Mutagenicity test of Ester Gum 8BG in the rat primary hepatocyte unscheduled DNA synthesis assay. Unpublished Report No. 10349-0-447 from Hazleton Laboratories America, Inc., Kensington, MD. Submitted to WHO by Hercules Inc., DE. Conner AH, 1989. Chemistry of other components in naval stores. In Naval Stores. DF Zinkel and J Russell (Eds). Pages 440-475. Pulp Chemicals Association, Inc., New York. Downs AMR and Sansom JE, 1999. Colophony allergy: a review. Contact Dermatitis 241, 305-310. EFSA (European Food Safety Authority), 2009. Guidance on Safety assessment of botanicals and botanical preparations intended for use as ingredients in food supplements. EFSA Journal 7(9) 1249. Federal Register, March 29, 2005. Vol. 70, No. 59, 15756-15758. Federal Register, August 22, 2007. Vol. 72, No. 162, 46895-46896. Food Chemicals Codex 6th ed., 2008. Gaefvert ELP, Shao AT, Karlberg U, Nilsson and Nilsson JLG, 1994. Allergenicity of rosin (colophony) esters: (II). Glycerol monoabeitate identified as contact allergen. Contact Dermatitis 31, 1117. EFSA Journal 2010; 8(7):1654 26 The use of glycerol esters of gum rosin as a food additive Geier J and Hausen BM, 2006. Epikutantestung mit chemisch modifiziertem Kolophonium. Akt. Dermatol. Vol. 32, 239-242 Gupta G, Forsyth A, 1999. Allergic contact reactions to colophony presenting as oral disease. Contact Dermatitis, Vol. 40, 332-333. Hausen BM, 1998. The sensitizing capacity of ginkgolic acids in guinea pigs. American Journal of Contact Dermatitis Vol 9, Issue 3, 146-148. Hausen BM, Kuhlwein A, Schulz KH, 1982. Kolophonium-Allergie. Dermatosen Vol. 30, Issue 4, 107115. Hausen BM and Mohnert J, 1989. Contact allergy to colophony: (V). Patch test results with different types of colophony and modified colophony products. Contact Dermatitis 20, 295-301. Illing HPA, Malmfors T, Rodenburg L, 2009. Skin sensitization and possible groupings for “read across” for rosin based substances. Regulatory Toxicology and Pharmacology Vol. 54, Issue 3, 234-241. IPCS (International Programme on Chemical Safety), 1987. Principles for the safety assessment of food additives and contaminants in food. World Health Organization, Geneva, Switzerland, Environmental Health Criteria 70. IPCS (International Programme on Chemical Safety), 1990. Principles for the toxicological assessment of pesticide residues in food. World Health Organization, Geneva, Switzerland, Environmental Health Criteria 104. Ishidate M Jr., Sofuni T, Yoshikawa K, Hayashi M, Nohmi T, Sawada M and Matsuoka A, 1984. Primary mutagenicity screening of food additives currently used in Japan. Food and Chemical Toxicology 22, 623-636. JECFA (Joint FAO/WHO Expert Committee on Food Additives), 1996a. Toxicological Evaluation of Certain Food Additives. WHO Food Additive Series 35, forty-fourth meeting. World Health Organization, Geneva, Switzerland. JECFA (Joint FAO/WHO Expert Committee on Food Additives), 1996b. Toxicological Evaluation of Certain Food Additives. WHO Food Additive Series 37, forty-sixth meeting. World Health Organization, Geneva, Switzerland. JECFA (Joint FAO/WHO Expert Committee on Food Additives), 1997. Evaluation of Certain Food Additives and Contaminants. WHO Technical Report Series 868. Forty-six report of the Joint FAO/WHO Expert Committee on Food Additives. World Health Organization, Geneva, Switzerland. EFSA Journal 2010; 8(7):1654 27 The use of glycerol esters of gum rosin as a food additive JECFA (Joint FAO/WHO Expert Committee on Food Additives), 2006. Combined Compendium of Food Additives Specifications, FAO JECFA Monographs 1. (Last updated web version: July 2009). JECFA, (Joint FAO/WHO Expert Committee on Food Additives), 2009, 71st meeting, Compendium of Food Additive Specifications, FAO JECFA Monographs 7. JECFA (Joint FAO/WHO Expert Committee on Food Additives), 2010. Safety Evaluation of Certain Food Additives. WHO Food Additive Series 62, seventy-first meeting. World Health Organization, Geneva, Switzerland. Joye M, Proveaux AT, Lawrence RV, 1973. Composition of neutral oils from rosin, J. Am. Oil Chem. SoHic. 52, 334. Karlberg A-T, Bergstedt E, Boman A, Bohlinder K, Lidén C, Lars J, Nilsson G, Wahlberg JE, 1985. Is abietic acid the allergenic component of colophony? Contact Dermatitis 13, 209 – 215. Karlberg AT, Basketter D, Goossens A and Lepoittevin JP, 1999. Regulatory classification of substances oxidized to skin sensitizers on exposure to air. Contact Dermatitis 40, 183-188. Kay JH, 1960a. Ninety-day subacute oral toxicity of gum rosin. Unpublished report from Industrial BioTest Laboratories, Inc., Northbrook, IL. Report to Hercules Powder Company, Inc. Kay JH, 1960b. Ninety-day subacute oral toxicity of N-wood rosin. Unpublished report from Industrial Bio-Test Laboratories, Inc., Northbrook, IL. Report to Hercules Powder Company, Inc. (Cited in JECFA 1996a). Kay JH, 1960c. Ninety-day subacute oral toxicity of Ester Gum 8D. Unpublished report from Industrial Bio-Test Laboratories, Inc., Northbrook, IL. Report to Hercules Powder Company, Inc. (Cited in JECFA 1996a). Kohn FE, 1962a. Two-year chronic oral toxicity of gum rosin-albino rats. Unpublished report from Industrial Bio-Test Laboratories, Inc., Northbrook, IL. Report to Hercules Powder Company, Inc. Kohn FE, 1962b. Two-year chronic oral toxicity of gum rosin-dogs. Unpublished report from Industrial Bio-Test Laboratories, Inc., Northbrook, IL. Report to Hercules Powder Company, Inc. Kohn FE, 1962c. Two-year chronic oral toxicity of N-wood rosin-albino rats. Unpublished report from Industrial Bio-Test Laboratories, Inc., Northbrook, IL. Report to Hercules Powder Company, Inc. Kohn FE, 1962d. Two-year chronic oral toxicity of N-wood rosin-dogs. Unpublished report from Industrial Bio-Test Laboratories, Inc., Northbrook, IL. Report to Hercules Powder Company, Inc. EFSA Journal 2010; 8(7):1654 28 The use of glycerol esters of gum rosin as a food additive Lin T-H, 1996. Metabolism study of Ester Gum 8BG in human fecal extracts and simulated human gastric juice. Report Project No. 8871 from Southern Research Institute, Birmingham, AL. Submitted to WHO by ILSI North America, Washington, D.C. Mukherjee A, Agarwal K and Chakrabarti J, 1992. Genotoxicity studies of the food additive ester gum. F. Chem. Tox. 30, 627-630. Murli H, 1988. Mutagenicity test on Ester Gum 8BG OSR in an in vitro cytogenetic assay measuring chromosomal aberration frequencies in Chinese hamster ovary (CHO) cells. Unpublished Report No. 10349-0-437 from Hazleton Laboratories America, Inc., Kensington, MD. Submitted to WHO by Hercules Inc., DE. NACGM/EACGI, 1996, National Association of Chewing Gum Manufacturers and European Association of the Chewing Gum Industry. NACGM and EACGI White Paper Reviewing Safety Information on Chewing Gum Base Ingredients and Responding to Issues Raised in United Kingdom Food Advisory Committee’s Report on Chewing Gum Base, June 5, 1996. Nasirullah, Krishnamurthy MN and Nagaraja KV, 1995. Detection and determination of ester gum (substitute for brominated vegetable oil) in ready-to-serve beverages and their concentrates. J. Food Sci. Technol. Vol. 32, Issue 3, 240-242. Nestmann ER, Lee EG, Mueller JC and Douglas GR, 1979. Mutagenicity of resin acids identified in pulp and paper mill effluents using the Salmonella/mammalian-microsome assay. Environ. Mutagen. 1, 361-369. Nestmann ER and Lee EG, 1983. Mutagenicity of constituents of pulp and paper mill effluent in growing cells of Saccharomyces cerevisiae. Mutat. Res. 119, 273-280. Noker PE, 1996. Pharmacokinetic study of Ester Gum 8BG in rats. Report project No. 8801 from Southern Research Institute, Birmingham, AL. Submitted to WHO by ILSI North America, Washington, D.C. Radomski JL, 1965. The absorption, fate and excretion of dehydroabietic acid, isopimaric acid and tetrahydroabietic acid in rats. Unpublished report (no Study No. given) from University of Miami School of Medicine, Coral Gables, FL, USA. Submitted to WHO by Hercules Inc., Wilmington, DE, USA (Cited in JECFA 1996a). Satyawan I, Oranje AP. and van Joost T, 1990. Perioral dermatitis in a child due to rosin in chewing gum. Contact Dermatitis, 22: 182-183. SCF (Scientific Committee for Food), 1994. Reports of the Scientific Committee for Food (32nd series), opinion expressed on 19 June 1992. Revision. http://ec.europa.eu/food/fs/sc/scf/reports/scf_reports_32.pdf EFSA Journal 2010; 8(7):1654 29 The use of glycerol esters of gum rosin as a food additive Shao LP, Gaefvert E, Karlberg AT, Nilsson U and Nilsson JLG, 1993. The allergenicity of glycerol esters and other esters of rosin (colophony). Contact Dermatitis. 28, 229-234. Soltes EJ and Zinkel DF, 1989. Chemistry of rosin. In Naval Stores. DF Zinkel and J Russell (Eds). Pages 261-330. Pulp Chemicals Association, Inc., New York. Weissmann G, 1986. Production, composition and properties of Chinese gum rosin and turpentine. Natural resources and development 23, 102. EFSA Journal 2010; 8(7):1654 30 The use of glycerol esters of gum rosin as a food additive GLOSSARY/ABBREVIATIONS ADI Acceptable Daily Intake ANS Scientific Panel on Food Additives and Nutrient Sources added to Food CAS Chemical Abstract Service CCFA Codex Committee on Food Additives CFR Code of Federal Regulations EC European Commission EFSA European Food Safety Authority FDA US Food and Drug Administration FTIR Fourier Transform Infrared GC/MS Gas Chromatography-Mass Spectrometry GEGR Glycerol Esters of Gum Rosin GETOR Glycerol Esters of Tall Oil Rosin GEWR Glycerol Esters of Wood Rosin GI Gastrointestinal INS International Numbering System IR Infrared spectroscopy JECFA Joint FAO/WHO Expert Committee on Food Additives (JECFA) NACGM/EACGI National Association of Chewing Gum Manufacturers and European Association of the Chewing Gum Industry NOAEL No-Observed-Adverse-Effect Level NMR Nuclear Magnetic Resonance SCF Scientific Committee on Food USDA US Department of Agriculture VOC Volatile Organic Compounds EFSA Journal 2010; 8(7):1654 31