ATOMS, MOLECULES & ELECTRONIC STRUCTURE
advertisement

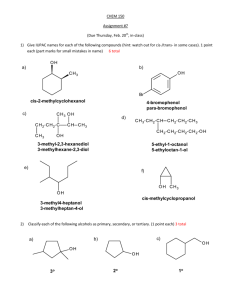
6. THE HALOGENS 1. (ii) electron affinity / kJ mol-1 (iii) heat of dissociation / kJ mol-1 (iv) boiling point / °C (v) boiling point / °C F 1685 F -335 F2 159 F2 -187 HF 19 Cl 1255 Cl -381 Cl2 239 Cl2 -35 HCl -115 Br 1142 Br -331 Br2 188 Br2 59 HBr -67 I 1010 I -305 I2 147 I2 183 HI -51 (84-II 7(b) / 8 marks) 2. (ii) Unlike the other halogens, fluorine exhibits only one oxidation state in its compounds. (86-II 4(a) / 3 marks) Explain the following facts : (i) The boiling points of the halogens increase as the group is descended. (ii) The bond dissociation energy of F2 is less than that of Cl2. 4. 7. (i) Compare the chemistry of chlorine, bromine and iodine in their reactions with an alkali. (ii) Under what condition will bromide solution react with bromate(V) solution ? (87-II 4(a) / 6 marks) 8. Compare the reaction of aqueous alkali with each of fluorine, chlorine, bromine and iodine (i) at 295 K ; (ii) at 350 K. (93-II 6(a) / 5 marks) 9. Give the formulae of two chlorine-containing salts in which the oxidation numbers of Cl are +1 and +5 respectively. Suggest one common chemical property shown by both compounds. (91-I 3(c) / 2 marks) 10. What is the highest oxidation state of iodine ? Give an example of an iodine-containing compound in which iodine is in this oxidation state. (94-II 4(c) / 2 marks) (iii) Iodine is more soluble in aqueous potassium iodide solution than in water. (95-II 4(a) / 6 marks) 11. Write the formulae of three oxoacids of chlorine, and arrange them in order of increasing acid strength. (94-I 2(c)(iii) / 2 marks) (i) Why is the Cl-Cl bond stronger than the F-F or Br-Br bonds ? 12. Complete and balance the following equations : (ii) Explain what is meant by ‘disproportionation’, and write an equation involving a compound of chlorine to illustrate your answer. (91-II 4(c) / 4 marks) 5. (iii) Write a balanced equation for the reaction between BrO3-(aq) and Br-(aq) in acidic solution to give Br2(aq). (94-I 2(a) / 4 marks) Account for the following facts : (i) Fluorine is the most reactive non-metal. 3. (ii) Write a balanced equation, involving the reaction of chlorine with KOH(aq), to illustrate a disproportionation. Briefly explain the variations and anomalies in the properties shown below : (i) ionization energy / kJ mol-1 (i) Distinguish between the terms ‘redox reaction’ and ‘disproportionation’. What is meant by a disproportionation reaction ? Show, with the help of a balanced equation, that the reaction of bromine with sodium hydroxide solution is such a reaction. (85-I 2(d) / 3 marks) room temp → H2O + (i) H2SO4 + KBr (conc.) heat → (ii) H3PO4 + KBr (conc.) + + + room temp → H2O + (iii) NaOH + Cl2 + (92-I 3(f) / 3 marks) 13. What would be observed when concentrated sulphuric acid is gently heated with (i) solid sodium chloride ? (ii) solid sodium bromide ? Explain your answers and write balanced equations for the reactions involved. (87 CE 1(c) / 6 marks) 14. Write the equation in each case, for the reaction of concentrated H3PO4 with NaCl and NaBr. Will concentrated H2SO4 give a similar reaction in each case ? Explain any difference(s) in reactivity between concentrated H3PO4 and concentrated H2SO4. (93-II 6(c) / 3 marks) 15. Write balanced equation(s) for the reaction between (i) concentrated H2SO4 and NaCl, and (ii) concentrated H2SO4 and NaI, and comment on the difference. 20. Devise an experiment to distinguish between KBr(s) and a mixture containing approximately 40% KCl(s) and 60% KBr(s) by mass. (95-I 4(a) / 3 marks) 21. Consider the data given below for the hydrogen halides and answer the questions that follow. Standard enthalpy change of formation / kJ mol-1 H-F H-Cl H-Br H-I -269.4 -92.8 -36.8 +26.1 bond dissociation energy / kJ mol-1 +562 +430 +367 +298 (94-II 4(d) / 3 marks) (i) Explain briefly the trend in the bond dissociation energy of the hydrogen halides. 16. For each of the following, state the expected observation and write the relevant balanced equation(s). (i) KIO3(aq) is added to acidified KI(aq) (ii) KBr(s) is heated with concentrated H2SO4. (99-I 3(c) / 4 marks) (ii) At temperatures above 400 K, hydrogen iodide decomposes to produce violet fumes, but, hydrogen chloride and hydrogen bromide do not decompose. Briefly explain this difference and write a balanced equation for the decomposition of HI. (95-II 4(c) / 7 marks) 17. (i) How do boiling points of the hydrogen halides vary down the halogen group ? Account for the variation. (ii) Comment on the difference between hydrogen fluoride and the other hydrogen halides with regard to their acid strengths in water. How does this difference arise ? (86-II 4(b) / 6 marks) 18. Explain why the acidity of 0.1 M HF(aq) is weaker than that of 0.1 M HCl(aq). (94-I 2(c)(iv) / 2 marks) 22. Write an essay to describe the typical reactions of halogens and the uses of halides. (92 essay / 20 marks) 23. Briefly describe how a sample of dry chlorine gas can be prepared in the laboratory. Draw a labeled diagram of the laboratory set-up and state the safety precaution(s) that is/are required. (97-I 7(a) / 6 marks) 19. Explain why THE END (i) the boiling point of HF is higher than that of HCl; (ii) the boiling point of HI is higher than that of HBr. (97-I 1(d) / 2 marks) Solutions to Halogens 1. (i) increase in size along the series ⇒ decreasing attraction on the outermost e- ⇒ decreasing I.E. 1 (ii) maximum at chlorine (1) increase in size ⇒ decreasing tendency to attract e- ⇒ smaller E.A. down the series (2) in the smallest F atom, addition of an e- introduces severe repulsion among lone-pair electrons 1 1 (iii) maximum at chlorine (1) smaller attraction on bonding (bond-pair) electrons with increasing size (or increasing bond length) ⇒ smaller bond energy down the series (2) greatest repulsion among non-bonding (lone-pair) electrons in fluorine 2. 3. 4. 5. 6. 1 (iv) large size ⇒ large van der Waals’ forces ⇒ increasing b.p. down the series 1 (v) larger van der Waals’ forces down the group ⇒ increasing b.p. down the series strong hydrogen bonding in HF explains its abnormal high b.p. 1 1 (8) (i) (1) weak F-F bond strength (2) high bond strength between fluorine and the other elements in covalent compounds (3) small size of fluoride ion means high lattice energy (or hydration energy) (Do not accept F is the most electronegative) (any 2; 1 mark each) (ii) fluorine has no vacant and low-lying d-orbitals to expand its oxidation state (octet) 1 (i) b.p. of halogens depend on the strength of their intermolecular forces (van der Waal’s forces) which is related to the strength of the instantaneous (temporary) dipole of the molecule. Descending the group, with the increase in molecular size, the strength of the instantaneous dipole increases. Hence more energy is needed for the boiling of the higher members. ½ ½ ½+½ (ii) Extremely short F-F distance leads to high repulsion due to lone pair electrons of fluorine atoms. Therefore F-F bond is weaker than expected. 1+1 (iii) I2 forms soluble complex I3- with KI in solution. Therefore, I2 appears to be more soluble. I2(s) + I (aq) ⇔ I3 (aq) 1 1 (i) Cl-Cl > Br-Br because weaker attraction on bonding electrons with increasing atomic size Cl-Cl > F-F because greatest repulsion among non-bonding electrons in fluorine weakens the F-F bond 1 1 (ii) Disproportionation is a change in which the same species is both oxidized and reduced at the same time. 3 NaClO → 2 NaCl + NaClO3 1 1 In a reaction, the same substance is both oxidized and reduced. 3 Br2 + 6 OH → 5 Br + BrO3 + 3 H2O Br2 is reduced to Br ; oxidation number of Br decreases from 0 to -1 Br2 is oxidized to BrO3 ; oxidation number of Br increases from 0 to +5 OR : Br2 + 2 OH → Br + BrO + H2O Br2 is reduced to Br ; oxidation number of Br decreases from 0 to -1 Br2 is oxidized to BrO ; oxidation number of Br increases from 0 to +1 1 1 (i) redox : one species is oxidized, and another is reduced disproportionation : single species is simultaneously oxidized and reduced 1 1 1 or 0 (ii) or : 7. 1 Cl2 + 2 KOH → KCl + KOCl + H2O 3 Cl2 + 6 KOH → 5 KCl + KClO3 + 3 H2O - 1 (must be balanced) - (i) All halogens undergo disproportionation in alkali to XO and then to XO3 1 fast slow - - - X2 + 2 OH → X + XO + H2O 3 XO → 2 X + XO3 1 Rate of 2nd reaction depends on temperature and concentration of alkali for chlorine : form ClO in cold dilute alkali / form ClO3 in hot conc. alkali for bromine : little BrO , mainly BrO3 for iodine : only IO3 and I (ii) acidic medium 8. (i) At 295K, Cl2, Br2 and I2 disproportionate according to the equation : X2 + 2 OH → X + XO + H2O BrO and IO are unstable and will undergo further disproportionate : X = Br or I 3 XO ⇔ 2 X + XO3 F2 is the strongest oxidizing agent, it will not disproportionate. Instead, it will oxidize the OH ion (ii) At 350K, Cl2, Br2 and I2 all disproportionate as follows : 3 X2 + 6 OH → 5 X + XO3 + 3 H2O F2 oxidizes the alkali to form O2 : 2 F2 + 4 OH → 4 F + O2 + 2 H2O 9. +1 : NaClO +5 : KClO3 Both are oxidizing agents. 1 2 1 (6) 1 1 1 1 1 (5) 10. +7 , KIO4 11. HClO < HClO2 < HClO3 < HClO4 (1 mark for 3 formulae 1 mark for order of acidity) 12. (i) 3 H2SO4 + 2 KBr → 2 H2O + 2 KHSO4 + Br2 + SO2 (ii) H3PO4 + KBr → HBr + KH2PO4 (iii) 2 NaOH + Cl2 → H2O + NaCl + NaClO @ 1 mark 13. (i) White fumes are formed. Explanation -- H2SO4 reacts with Cl to form HCl, a volatile acid H2SO4 + NaCl → HCl + NaHSO4 1 1 1 (ii) Reddish brown fumes are formed. Explanation – conc. H2SO4 behaves as an oxidizing agent when it reacts with Br to form brown Br2 gas H2SO4 + NaBr → HBr + NaHSO4 H2SO4 + 2 HBr → Br2 + SO2 + 2 H2O (or : 3 H2SO4 + 2 NaBr → 2 NaHSO4 + Br2 + SO2 + 2 H2O ) 14. Reaction between NaX and H3PO4 (X = Cl or Br) H3PO4 + NaX → HX + NaH2PO4 With H2SO4 , NaCl gives similar reaction, while NaBr will react to give also Br2 and SO2 H2SO4 + 2 HBr → Br2 + SO2 + 2 H2O c. H2SO4 is an oxidizing agent while c. H3PO4 is not 15. (i) H2SO4 + NaCl → HCl + NaHSO4 (ii) H2SO4 + NaI → HI + NaHSO4 8 HI + H2SO4 → 4 I2 + H2S + 4 H2O HI, being a stronger reducing agent than HCl, will be oxidized by c. H2SO4 to give I2 16. (i) A brown solution is formed IO3- + 6 H+ + 5 I- → 3 I2 + 3 H2O 1 1 1 (6) 1 1 1 (3) 1 1 1 (3) 1 1 (ii) Reddish brown solution is formed / a gas with a choking smell evolves KBr + H2SO4 → KHSO4 + HBr 2 HBr + H2SO4 → Br2 + 2 H2O + SO2 1 ½ ½ 17. (i) b.p. increases down the group except for HF, which has anomalously high b.p. b.p. increases down the group due to an increase in van der Waals’ forces between molecules with increase in size b.p. of HF is higher due to the presence of stronger H-bond 1 1 (ii) HF in water behaves as a weak acid while other hydrogen halides in water form strong acidic solutions The very high H-F bond energy is the reason why ionization of HF in water is not extensive. 1 1 1 1 18. HF is extensively hydrogen-bonded in water so that H-F is stronger than H-Cl HF is only partly ionized and HCl is completely ionized in water 1 1 19. (i) The intermolecular attraction between HF molecules is H-bond which is stronger than the van der Waals’ forces between HCl molecules (ii) The intermolecular forces between HI and between HBr are both dipole-dipole attraction/van der Waals’ forces. HI has a greater no. of e-, thus it can exert a stronger attraction between molecules. ½ + ½ 20. Prepare strong aqueous solution Add AgNO3 to solution in test tube, let the precipitate settle Measure height of precipitate after decanting solution Add dil. NH3 to precipitate, decant and measure height again ½ ½ + ½ ½ ½ ½ 21. (i) Trend : bond dissociation energy decreases with increasing molecular mass of hydrogen halides Explanation : The H-X bond lengths increase as the atomic radii of the halogens increase The longer the bond, the weaker it is and the bond dissociation energy is smaller 1 1 1 (ii) HI has the smallest bond energy, the activation energy for its decomposition is the lowest and therefore it is the most easily decomposed HX. However, HBr and HCl do not decompose at temperatures > 400K From the ∆Hθf values, HI is the most unstable hydrogen halide with respect to decomposition to its elements. 2 HI(g) → H2(g) + I2(g) 22. A. Typical Reactions of Halogens (max. 7 marks) Mentioning the typical reactions of halogens are due to their oxidizing powers / their positions as Group VII elements making them readily accept an electron ½ ½ 1 1 1 1 1 Mentioning that the chemistry of fluorine is anomalous when compared to other halogens, so it does not carry out many of the typical reactions (e.g. reaction with water / alkali) (1) Reactions as oxidizing agents 1. Reactions with metals 2. Reactions with non-metals 3. Reactions with hydrogen 4. Reactions with other reducing agents (e.g. Fe2+, SO2, NH3) 5. Displacement reactions of the halides Y2 + 2 X- → 2 Y- + X2 (Y is higher than X) Disproportionation reactions (except for fluorine) Reactions with organic compounds e.g. with alkanes (free-radical substitution) ; with alkenes (electrophilic addition) B. Uses of Halides (max. 3 marks) 23. Add conc. HCl (dropwise) to MnO2 and heat the mixture Pass the gas produced through water to remove HCl(g) and then through conc. H2SO4 to remove water vapour Collect by downward delivery / in a gas syringe (1) (1) (1) (1) (1) 1 (max. 2) (@ ½ each) 1 ½+½ Precaution : carry out the experiment in fume cupboard (Also accept preparation using conc. HCl and KMnO4 ; action of acid on bleach) ½ ½ Marks fpr the labeled diagram of the set-up 3 (½ mark for tap funnel; ½ mark for labelling the reagents; 1 mark for correct set-up for washing the gas with water / c. H2SO4 ; 1 mark for collection of dry Cl2 )