Health Benchmarks® Clinical Quality Indicator

Client
Measure Title
Disease State
Health Benchmarks
®
Clinical Quality Indicator Specification 2007
HMSA: PQSR 2007
ANNUAL CREATININE & POTASSIUM TESTS FOR PRE-ESRD PATIENTS
Renal Disease Indicator Classification
1
Disease
Management
C Strength of
Recommendation
2
Clinical Intent
Physician
Specialties
Clinical Rationale
To ensure that all eligible members identified as having pre-esrd receive a creatinine and potassium test within a clinically appropriate timeframe.
Refer to PQSR 2007 Specialty Matrix
Disease Burden
•
Approximately 20 million American adults have kidney disease.[1]
•
The number of patients treated with dialysis or transplantation is expected to increase from 340,000 in 1999 to 651,000 in 2010.[2]
Reason for Indicated Intervention or Treatment
•
In order to monitor disease progression and to assess the effect of interventions to slow the GFR decline in patients with chronic kidney disease, it is important to assess the rate of Glomerular Filtration Rate
(GFR) decline.[3]
•
In most cases, GFR estimation using a prediction equation that takes the serum creatinine level into account [4, 5] is just as reliable as assessing
GFR by creatinine clearance measurement using a timed urine collection.[6]
•
Hyperkalemia is a common disorder in patients with kidney disease, especially when the GFR falls below 20 ml/min, and is frequently exacerbated by the use of drugs such as angiotensin-converting enzyme inhibitors and aldosterone receptor blockers.[7]
Evidence supporting Intervention or Treatment
•
There are no true randomized studies examining patient survival benefits with early versus late initiation of dialysis, but the IDEAL study is an ongoing trial designed to investigate whether the timing of dialysis initiation affects survival.[8]
•
The current literature, consisting of one prospective cohort study, a retrospective study and a cost-benefit analysis, offers conflicting evidence with regard to the effect of early dialysis initiation on survival.[9-
11] One comparative study found an association between increased mortality and initiating dialysis therapy at greater GFRs. [12]
•
An analysis of 14,722 adults from the Third National Health and Nutrition
Examination Survey (NHANES III) showed changes in the mean serum potassium concentration with relatively modest creatinine clearance reductions (around 50 to 60 ml/min).[13]
•
While there is no evidence showing that periodic monitoring of potassium levels improves patient morbidity or mortality, patients with chronic kidney disease are at higher risk for developing hyperkalemia, and may benefit from early detection of asymptomatic increased potassium levels.
© 2007 Health Benchmarks®
Confidential and Proprietary
All Rights Reserved
585.esrdtest v 4.0
Measure: esrdte585
[14-18]
Recommendations
•
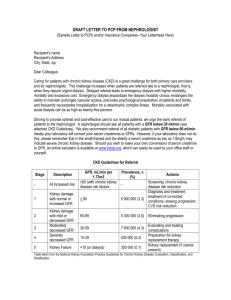
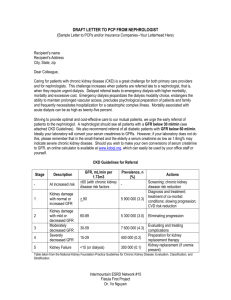
The National Kidney Foundation recommends obtaining serum creatinine measurements for GFR estimation at least yearly in patients with chronic kidney disease.[3]
•
The Department of Veterans Affairs/Veterans Health Administration recommends periodically checking potassium levels in patients with chronic kidney disease.[7]
Source
Denominator
Definition
Denominator
Exclusion Definition
Health Benchmarks, Inc.
Continuously enrolled members who had at least two diagnoses of chronic kidney disease during the year prior to the measurement year.
Relevant Billing Codes:
ICD-9 diagnosis code(s): 585, 585.1-585.5, 585.9
Members with at least one diagnosis of or evidence of end stage renal disease
(ESRD), identified in the measurement year or year prior.
Relevant Billing Codes:
ICD-9 diagnosis code(s): 585.6
Numerator
Definition
ICD-9 status “V” code(s): V45.1x, V56.xx
CPT-4 code(s): 36800, 36810, 36815, 36819-36821, 36825, 36830-
36833, 36838, 36145, 90918-90925, 90935-90999
Members who received at least one creatinine and one potassium test 1-365 days after the index date.
Relevant Billing Codes:
CPT-4 code(s): 80048, 80050, 80051, 80053, 80069, 82565, 82575,
84132
High score implies better performance Interpretation of
Score
Physician
Attribution
Description
References
Score all physicians (in the selected specialties) who saw the member 0-365 days after the index date
1.
2.
3.
United States Renal Data System. USRDS 2004 Annual Data Report.
Bethesda, MD: National Institute of Diabetes and Digestive and Kidney
Diseases, National Institutes of Health (NIH), DHHS. www.usrds.org
.
2002.
Excerpts from the United States Renal Data System's 2000 annual data report: atlas of end-stage renal diseases: renoprotective benefits of renin-angiotensin system inhibition.
Am J Kidney Dis, 2000. 36 (6 suppl
2): p. S1-S279.
Grodum, K., A. Heijl, and B. Bengtsson, A comparison of glaucoma
© 2007 Health Benchmarks®
Confidential and Proprietary
All Rights Reserved
585.esrdtest v 4.0
Measure: esrdte585
4.
5. patients identified through mass screening and in routine clinical practice.
Acta Ophthalmol Scand, 2002. 80 (6): p. 627-31.
Levey, A.S., et al., A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation.
Modification of Diet in Renal Disease Study Group.
Ann Intern Med,
1999. 130 (6): p. 461-70.
Cockcroft, D.W. and M.H. Gault, Prediction of creatinine clearance from serum creatinine.
Nephron, 1976. 16 (1): p. 31-41.
7. adults with chronic renal failure.
Am J Kidney Dis, 1998. 32 (1): p. 23-31.
Management of Chronic Kidney Disease and Pre-ESRD in the Primary
Care Setting.
November 2000, VA/DoD Evidence-Based Clinical Practice
Guideline Working Group, Veterans Health Administration, Department of Veterans Affairs, and Health Affairs, Department of Defense. Office of
8.
Quality and Performance. publication 10Q-CPG/ESRD-00: Washington,
DC.
Cooper, B.A., et al., The Initiating Dialysis Early and Late (IDEAL) study: study rationale and design.
Perit Dial Int, 2004. 24 (2): p. 176-81.
9. Traynor, J.P., et al., Early initiation of dialysis fails to prolong survival in patients with end-stage renal failure.
J Am Soc Nephrol, 2002. 13 (8): p.
2125-32.
10. Korevaar, J.C., et al., When to initiate dialysis: effect of proposed US guidelines on survival.
Lancet, 2001. 358 (9287): p. 1046-50.
11. Bonomini, V., et al., Benefits of early initiation of dialysis.
Kidney Int
Suppl, 1985. 17 : p. S57-9.
12. Kazmi, W.H., et al., Effect of comorbidity on the increased mortality associated with early initiation of dialysis.
Am J Kidney Dis, 2005. 46 (5): p. 887-96.
13. Hsu, C.Y. and G.M. Chertow, Elevations of serum phosphorus and potassium in mild to moderate chronic renal insufficiency.
Nephrol Dial
Transplant, 2002. 17 (8): p. 1419-25. disease. Part II: patients on dialysis (stage 5).
Int Urol Nephrol, 2004.
36 (3): p. 469-72.
© 2007 Health Benchmarks®
Confidential and Proprietary
All Rights Reserved disease (CKD), Part I: patients not on dialysis (stages 3-4).
Int Urol
Nephrol, 2004. 36 (3): p. 465-8.
16. Gennari, F.J. and A.S. Segal, Hyperkalemia: An adaptive response in chronic renal insufficiency.
Kidney Int, 2002. 62 (1): p. 1-9.
17. Ahmed, J. and L.S. Weisberg, Hyperkalemia in dialysis patients.
Semin
Dial, 2001. 14 (5): p. 348-56.
18. Kemper, M.J., E. Harps, and D.E. Muller-Wiefel, Hyperkalemia: therapeutic options in acute and chronic renal failure.
Clin Nephrol, 1996.
46 (1): p. 67-9.
585.esrdtest v 4.0
Measure: esrdte585
1
Indicator Classification (Adapted from Health Plan Employer Data Information Set (HEDIS®) technical specifications)
Diagnosis
Measures applicable to patients receiving diagnostic workups for a symptom or condition that delineate appropriate laboratory or radiological testing to be performed (e.g. evaluation of thyroid nodule; pregnancy test in patients with vaginal bleeding or abdominal pain)
Effectiveness of Care
Prevention
Screening
Measures applicable to asymptomatic individuals that are designed to prevent the onset of the targeted condition (e.g. immunizations).
Measures applicable to asymptomatic patients who have risk factors or preclinical disease, but in whom the condition has not become clinically apparent
(e.g. pap smears; screening for elevated blood pressure).
Disease
Management
Medication
Monitoring
Medication
Adherence
Utilization
Measures applicable to individuals diagnosed with a condition that are part of the treatment or management of the condition (e.g. cholesterol reduction in patients with diabetes; radiation therapy following breast conserving surgery; appropriate follow-up after acute event).
Measures applicable to patients taking medications with narrow therapeutic windows and / or potential preventable significant side effects or adverse reactions (e.g. thyroid stimulating hormone (TSH) testing after levothyroxine dose change; hepatic enzyme monitoring for patients using antimycotic pharmacotherapy)
Measures applicable to patients taking medications for chronic conditions that are designed to assess patient adherence to medication (e.g. adherence to lipid lowering medication).
Measures applicable to patients receiving treatment for a symptom or condition that advocate appropriate utilization of laboratory and pharmaceutical resources
(e.g. conservative use of imaging for low back pain; inappropriate use of antibiotics for viral upper respiratory infection).
© 2007 Health Benchmarks®
Confidential and Proprietary
All Rights Reserved
585.esrdtest v 4.0
Measure: esrdte585
2
Strength of Recommendation
Strength of Recommendation Based on a Body of Evidence
FIGURE 2. Algorithm for determining the strength of a recommendation based on a body of evidence
(applies to clinical recommendations regarding diagnosis, treatment, prevention, or screening). While this algorithm provides a general guideline, authors and editors may adjust the strength of recommendation based on the benefits, harms, and costs of the intervention being recommended. (USPSTF = U.S.
Preventive Services Task Force)
© 2007 Health Benchmarks®
Confidential and Proprietary
All Rights Reserved
585.esrdtest v 4.0
Measure: esrdte585
© 2007 Health Benchmarks®
Confidential and Proprietary
All Rights Reserved
585.esrdtest v 4.0
Measure: esrdte585