CHAPTER TEN MOLECULAR GEOMETRY MOLECULAR
advertisement

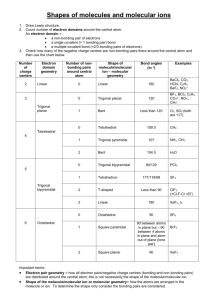
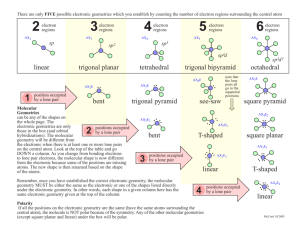
CHAPTER TEN CHEMICAL BONDING II: MOLECULAR GEOMETRY AND HYBRIDIZATION OF ATOMIC ORBITALS MOLECULAR GEOMETRY V S E P R VSEPR Theory In VSEPR theory, multiple bonds behave like a single electron pair MOLECULAR GEOMETRY Valence shell electron pair repulsion (VSEPR) model: Predict the geometry of the molecule from the electrostatic repulsions between the electron (bonding and nonbonding) pairs. Class # of atoms bonded to central atom # lone pairs on central atom AB2 2 0 Arrangement of electron pairs Molecular Geometry B B 1 Cl Be Cl MOLECULAR GEOMETRY Class # of atoms bonded to central atom # lone pairs on central atom Arrangement of electron pairs AB2 2 0 linear AB3 3 0 Molecular Geometry linear 2 MOLECULAR GEOMETRY Class # of atoms bonded to central atom # lone pairs on central atom AB2 2 0 linear linear trigonal planar trigonal planar AB3 3 0 AB4 4 0 Arrangement of electron pairs Molecular Geometry MOLECULAR GEOMETRY Class # of atoms bonded to central atom # lone pairs on central atom AB2 2 0 linear linear trigonal planar tetrahedral Arrangement of electron pairs AB3 3 0 trigonal planar AB4 4 0 tetrahedral AB5 5 0 Molecular Geometry 3 MOLECULAR GEOMETRY Class # of atoms bonded to central atom # lone pairs on central atom AB2 2 0 linear linear trigonal planar Arrangement of electron pairs Molecular Geometry AB3 3 0 trigonal planar AB4 4 0 tetrahedral tetrahedral trigonal bipyramidal trigonal bipyramidal AB5 5 0 AB6 6 0 4 MOLECULAR GEOMETRY Class # of atoms bonded to central atom # lone pairs on central atom AB3 3 0 AB2 E 2 1 Arrangement of electron pairs Molecular Geometry trigonal planar trigonal planar trigonal planar 5 MOLECULAR GEOMETRY Class # of atoms bonded to central atom # lone pairs on central atom AB4 4 0 tetrahedral AB3 E 3 1 tetrahedral Arrangement of electron pairs Molecular Geometry tetrahedral MOLECULAR GEOMETRY Class # of atoms bonded to central atom # lone pairs on central atom AB4 4 0 tetrahedral tetrahedral AB3 E 3 1 tetrahedral trigonal pyramidal AB2 E2 2 2 tetrahedral Arrangement of electron pairs Molecular Geometry O H H MOLECULAR GEOMETRY Class # of atoms bonded to central atom # lone pairs on central atom AB5 5 0 trigonal bipyramidal AB4 E 4 1 trigonal bipyramidal Arrangement of electron pairs Molecular Geometry trigonal bipyramidal 6 MOLECULAR GEOMETRY Class # of atoms bonded to central atom # lone pairs on central atom AB5 5 0 AB4 E 4 1 AB3 E2 3 2 Arrangement of electron pairs Molecular Geometry trigonal bipyramidal trigonal bipyramidal trigonal bipyramidal trigonal bipyramidal distorted tetrahedron F F Cl F MOLECULAR GEOMETRY Class # of atoms bonded to central atom # lone pairs on central atom AB5 5 0 AB4 E 4 1 AB3 E2 3 2 AB2 E3 2 3 I I Arrangement of electron pairs Molecular Geometry trigonal bipyramidal trigonal bipyramidal trigonal bipyramidal trigonal bipyramidal distorted tetrahedron T-shaped trigonal bipyramidal I MOLECULAR GEOMETRY Class # of atoms bonded to central atom # lone pairs on central atom AB6 6 0 octahedral AB5 E 5 1 octahedral Arrangement of electron pairs Molecular Geometry octahedral F F F Br F F 7 MOLECULAR GEOMETRY Class # of atoms bonded to central atom # lone pairs on central atom AB6 6 0 octahedral octahedral AB5 E 5 1 octahedral square pyramidal AB4 E2 4 2 octahedral Arrangement of electron pairs Molecular Geometry F F Xe F F 10.1 MOLECULAR GEOMETRY Predicting Molecular Geometry 1. Draw Lewis structure for molecule. 2. Count number of lone pairs on the central atom and number of atoms bonded to the central atom. 3. Use VSEPR to predict the geometry of the molecule. What are the molecular geometries of SO2 and SF4? 8 DIPOLE MOMENTS Bonds and molecules may be polar or nonpolar Relative to distribution of electrons Dipole moment (µ= Q x r) Bonds Molecule DIPOLE MOMENTS Dipole Moments and Polar Molecules electron poor region electron rich region H F δ+ δ- DIPOLE MOMENTS H2O vs CO2 BF3 vs NH3 cis-C2H2Cl2 vs trans- C2H2Cl2 9 DIPOLE MOMENTS H2O vs CO2 BF3 vs NH3 cis-C2H2Cl2 vs trans- C2H2Cl2 NH3 vs NF3 10.2 10 DIPOLE MOMENTS Does CH2Cl2 have a dipole moment? VALENCE BOND THEORY Change in electron density as two hydrogen atoms approach each other. 11 VALENCE BOND THEORY Covalent bond consists of pair of electrons of opposite spin within an AO Appears that to form bond, must have unpaired electron New AO--hybrid orbital Mix AO before bonding occurs Explains # of bonds and bond angles VALENCE BOND THEORY Hybridization – mixing of two or more atomic orbitals to form a new set of hybrid orbitals. 1. Mix at least 2 nonequivalent atomic orbitals (e.g. s and p). Hybrid orbitals have very different shape from original atomic orbitals. 2. Number of hybrid orbitals is equal to number of pure atomic orbitals used in the hybridization process. 3. Covalent bonds are formed by: a. Overlap of hybrid orbitals with atomic orbitals b. Overlap of hybrid orbitals with other hybrid orbitals 12 VALENCE BOND THEORY Draw Lewis Structure Count valence electron pairs (multiples = 1) # valence pairs = # hybrid orbitals (Table 10.4) VALENCE BOND THEORY Ground state orbital diagram (valence) Excitation Hybridization CH4, 13 What about NH3? 14 VALENCE BOND THEORY Ground state orbital diagram (valence) Excitation Hybridization BF3 , Formation of sp2 Hybrid Orbitals VALENCE BOND THEORY Ground state orbital diagram (valence) Excitation Hybridization BeCl2, 15 Formation of sp Hybrid Orbitals VALENCE BOND THEORY Ground state orbital diagram (valence) Excitation Hybridization SF6, HYBRIDIZATION OF MULTIPLE BONDS Extra electrons not located in hybrid orbitals Sigma Bond Pi Bond 16 HYBRIDIZATION OF MULTIPLE BONDS C2H4 (Lewis Structure) Each C is HYBRIDIZATION OF MULTIPLE BONDS C2H4 (Lewis Structure) Each C is 17 HYBRIDIZATION OF MULTIPLE BONDS C2H2 (Lewis Structure) Each C is HYBRIDIZATION OF MULTIPLE BONDS 18 HYBRIDIZATION OF MULTIPLE BONDS Sigma (σ) and Pi Bonds (π) Single bond Double bond Triple bond How many σ and π bonds are in the acetic acid (vinegar) molecule CH3COOH? σ bonds = π bonds = 19