Foul Water PreLab
advertisement
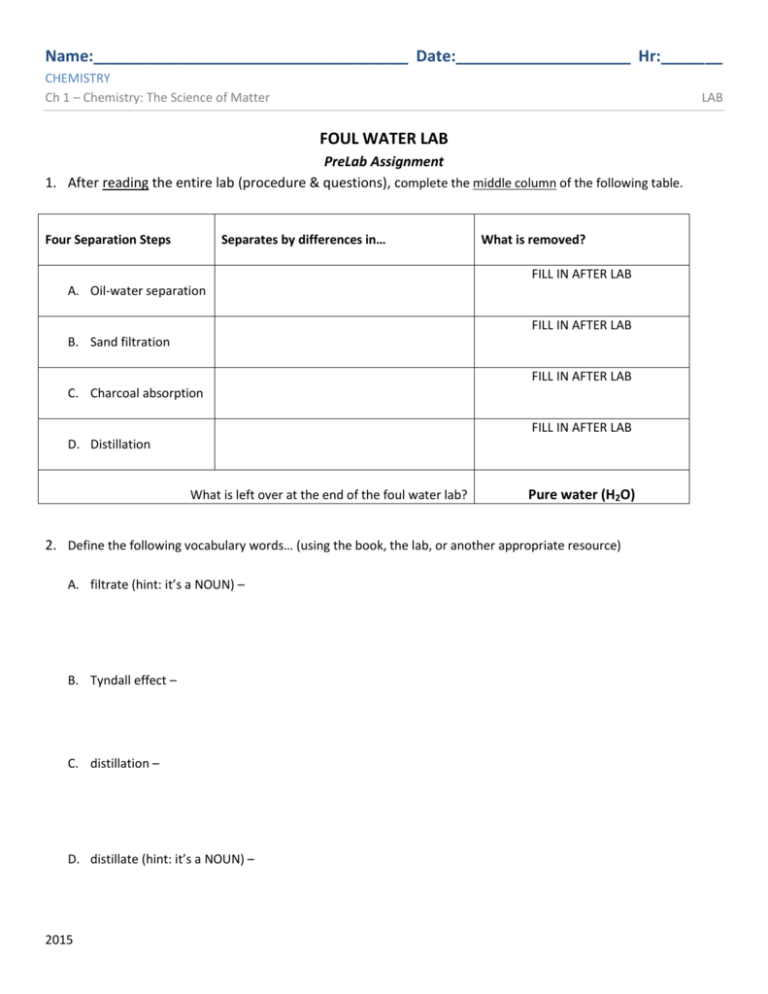
Name:____________________________________ Date:____________________ Hr:_______ CHEMISTRY Ch 1 – Chemistry: The Science of Matter LAB FOUL WATER LAB PreLab Assignment 1. After reading the entire lab (procedure & questions), complete the middle column of the following table. Four Separation Steps Separates by differences in… What is removed? FILL IN AFTER LAB A. Oil-water separation FILL IN AFTER LAB B. Sand filtration FILL IN AFTER LAB C. Charcoal absorption FILL IN AFTER LAB D. Distillation What is left over at the end of the foul water lab? Pure water (H2O) 2. Define the following vocabulary words… (using the book, the lab, or another appropriate resource) A. filtrate (hint: it’s a NOUN) – B. Tyndall effect – C. distillation – D. distillate (hint: it’s a NOUN) – 2015 Name:____________________________________ Date:____________________ Hr:_______ CHEMISTRY Ch 1 – Chemistry: The Science of Matter LAB FOUL WATER LAB Introduction The purification of water takes place constantly in nature. As water moves through the soil, the process of filtration can remove many of the impurities. Unfortunately there are many impurities found in our water systems that cannot be naturally removed. Purpose In this laboratory investigation, you will purify the provided foul water sample using three different separation techniques: oil-water separation, filtration, and charcoal absorption. You will also use macroscopic observation to record several qualitative and quantitative properties of the mixture throughout the investigation. Electrical conductivity and precipitation will also be observed. Your instructor will do an additional purification technique called distillation. Procedure PART I: Oil-Water Separation Because the oil and water layers of the foul water sample are immiscible (i.e. they don’t mix) and because they have different densities, these two portions of the foul water can be easily separated with a separatory funnel, like the one you will construct here. 1. First collect approximately 50 mL of the foul water from your instructor. Record the general characteristics of your water sample (color, clarity, odor, presence of oil, presence of solids, & volume) before treatment using Data Table 1 on your Data Sheet. Remember to record the volume to the appropriate number of decimal places. 2. Place a funnel in a clay triangle supported by an iron ring on a ring stand. A rubber tube has already been attached to the funnel. Use a pinch clamp to clamp the tube completely. 3. Swirl your foul water sample and then pour some of it into the funnel until the funnel is about ¾ full. Allow one minute for the sample to separate. Because oil is less dense than water, it will float on top, while the water (and anything dissolved in it) will sink to the bottom. 4. Carefully and slowly open the clamp to drain the lower layer into a clean 150mL beaker. Quickly reclamp the tube before the top layer reaches the bottom of the funnel. AT NO TIME SHOULD OIL GET INTO THE RUBBER TUBING!! 5. By picking up the funnel and dumping it out, drain the remaining oil layer into a second and separate waste beaker. DO NOT OPEN THE CLAMP TO DRAIN THE OIL LAYER!! 6. Repeat steps 3-5 until your entire original foul water sample has been separated. 7. Combine and SAVE all of the water (lower layers) that you have collected, discard only the oil layer in the sink with lots of tap water. 8. Record your observations on Data Table 1 on your Data Sheet for the after separation section. 9. Wash the funnel, rubber tubing, and oil waste beaker with soap and water. SAVE YOUR WATER!! Page 2 of 6 2015 Name:____________________________________ Date:____________________ Hr:_______ CHEMISTRY Ch 1 – Chemistry: The Science of Matter LAB PART II: Sand-Filtration Filtration is a separation technique that uses a porous material to separate a solid from a liquid. It works because large particles can be trapped in a filter while smaller ones can be allowed to pass through. You will often use filter paper to conduct filtrations, but for this part of the investigation, you will be using a sand-filter cup to simulate the filtration of run-off that occurs in nature and in water treatment facilities. 1. Obtain a pre-prepared sand-filter cup (a Styrofoam cup containing 1 cm of gravel, 2 cm of sand, and 1 cm of gravel on top). Place the cup onto a 150 mL (or 250 mL depending on its size) for filtering. DO NOT POUR OUT THE CONTENTS OF THE CUP INTO THE BEAKER!! 2. Gently pour your water sample from Part I into the cup. This process may be slow and will require patience. 3. Once the entire sample has been filtered through the sand-filter cup, record your new observations of this filtered water in the appropriate location on Data Table 1 on your Data Sheet. 4. SAVE your filtered water, but return the sand-filter cup on a folded paper towel. PART III: Charcoal Absorption Charcoal has the ability to attract and hold many different chemical compounds. This is particularly true of organic compounds. Charcoal is very porous and has an especially large surface area when in powdered form. A small amount of charcoal can hold onto an enormous amount of chemical impurities, even if they are dissolved in your “filtered” water sample from the previous step. 1. Obtain a single piece of filter paper and a funnel. Fold the paper as shown below and place it into the funnel. Wet the filter paper using a little bit of tap water on your finger. Place the funnel into a clay triangle and support it with a ring stand. Place a clean 150mL beaker underneath to collect your water. 2. Once step one is completed, ask your instructor to check your apparatus and add some activated charcoal to your filter paper. 3. Carefully and slowly pour the water sample through the funnel. You should try to keep the funnel/filter paper about ¾ full, but do not pour more water into the funnel than the level of the top of the filter paper or you will need to start this part of the lab all over again! 4. Collect the filtered water in the beaker. This water is called filtrate because it is the liquid that remains after a filtration step. 5. Record your observations in the appropriate location on Data Table 1 on your Data Sheet. Page 3 of 6 2015 Name:____________________________________ Date:____________________ Hr:_______ CHEMISTRY Ch 1 – Chemistry: The Science of Matter LAB Part IV: Electrical Conductivity Test This portion of the lab procedure is not a separation technique, but a test for the presence of electrically charged particles called ions. If you attempted to pass an electrical current through a sample of pure water (only H2O), no electrical current would travel through the water because no ions would be present. The ability of the electrical current to travel through the water increases as the number of dissolved charged particles increases. An electrical conductivity meter is used to measure the relative amount of current traveling through the water sample. 1. Obtain a conductivity meter. Use a dry paper towel to remove any moisture or materials collected on the metal probes of the meter. Be sure that your meter shows no conductivity when turned on, but shows high conductivity when turned on and the two probes are carefully touched together. There is a table on the back of the meter to help you interpret the results of this test. 2. Measure the conductivity of your filtrate from Part III. Record its relative conductivity (none, very low, medium, high, very high, etc.) on Data Table 2 on your Data Sheet. 3. Your instructor will test the conductivity of the sample collected after Part VI (Distillation), so be sure to add this conductivity to your Data Table 2 before leaving lab. Part V: Silver Nitrate Test Silver nitrate (AgNO3) is a compound with the ability to undergo a chemical change to react with chloride ions. Chloride ions are small electrically charged particles which dissolve easily in water and can pass through filters. If silver nitrate is added to a mixture containing chloride ions, a chemical reaction occurs that produces a white colored precipitate known as silver chloride (AgCl). A precipitate is an insoluble solid that forms from the reaction of two solutions. Precipitates are more dense than solutions, so they sink to the bottom of the container. WARNING: Silver nitrate solution is a toxic skin irritant. Use care when handling the bottle and avoid skin contact with the solution. 1. Add 2-3 drops of silver nitrate solution into your “purified” sample from Part IV. 2. Record your observations (“precipitate forms: positive chloride test” or “no reaction: negative chloride test”) on Data Table 2 on your Data Sheet. 3. Your instructor will perform this test on the sample collected after Part VI (Distillation), so be sure to add your observations of this test to your Data Table 2 on your Data Sheet before leaving lab. PART VI: Distillation Distillation is a separation technique which separates the components of a mixture by evaporation/boiling and subsequent condensation of its vapor. It works based on differences in the components’ boiling points. Because a boiling mixture does not increase in temperature until all of the boiling component is vaporized, the components can be collected one at a time. The liquid collected after distillation is called distillate. 1. In the appropriate location on your Data Sheet, make a sketch of the distillation apparatus. 2. Be sure to label the “mixture,” the “distillate,” the “condenser tube,” the “cooling water,” and the “heat source” also. Page 4 of 6 2015 Name:____________________________________ Date:____________________ Hr:_______ CHEMISTRY Ch 1 – Chemistry: The Science of Matter LAB FOUL WATER LAB Lab Partners: _________________________ _________________________ _________________________ DATA SHEET Data Table 1 – Observations – Be complete and descriptive. Record volumes to the appropriate decimal place! Color Clarity Odor Presence of oil Presence of solids Volume (mL) Part I: before treatment Part I: after separation Part II: after sandfiltration Part III: after charcoal absorption Data Table 2 – Tests Relative Electrical Conductivity Silver Nitrate Test Part IV: before distillation Part V: before distillation Part VI: after distillation Part VI: after distillation Labeled sketch of distillation apparatus (refer to Procedure PART VI for required labels list) Page 5 of 6 2015 Name:____________________________________ Date:____________________ Hr:_______ CHEMISTRY Ch 1 – Chemistry: The Science of Matter LAB QUESTIONS – Be clear, concise, and complete! Answer questions in your own words! 1. Calculate the percentage of the original water sample recovered after you have completed the third separation technique (after Part III). SHOW YOUR WORK!! 𝑉𝑜𝑙𝑢𝑚𝑒 𝑜𝑓 𝑝𝑢𝑟𝑖𝑓𝑖𝑒𝑑 𝑤𝑎𝑡𝑒𝑟 𝑃𝑒𝑟𝑐𝑒𝑛𝑡𝑎𝑔𝑒 𝑜𝑓 𝑃𝑢𝑟𝑖𝑓𝑖𝑒𝑑 𝑊𝑎𝑡𝑒𝑟 = × 100 𝑉𝑜𝑙𝑢𝑚𝑒 𝑜𝑓 𝐹𝑜𝑢𝑙 𝑊𝑎𝑡𝑒𝑟 𝑆𝑎𝑚𝑝𝑙𝑒 Your calculated percentage = __________ 2. Explain how the process of distillation works to separate mixture components. 3. List at least 2 reasons why distillation is not used to treat municipal water systems, like CWLP’s water. 4. Most aquarium water filters use activated charcoal. Explain specifically why charcoal is used to clean aquarium tanks? (Hint: consider your sample before and after charcoal absorption) 5. Explain why silver nitrate was used both before and again after the sample was distilled. 6. The electrical conductivity of a lake increases significantly in the early spring and then decreases by summer. This same increase is seen in late fall, then a decrease is observed for the winter. What causes this?? Page 6 of 6 2015






