Super Absorbent Polymer – Sodium Polyacrylate
advertisement
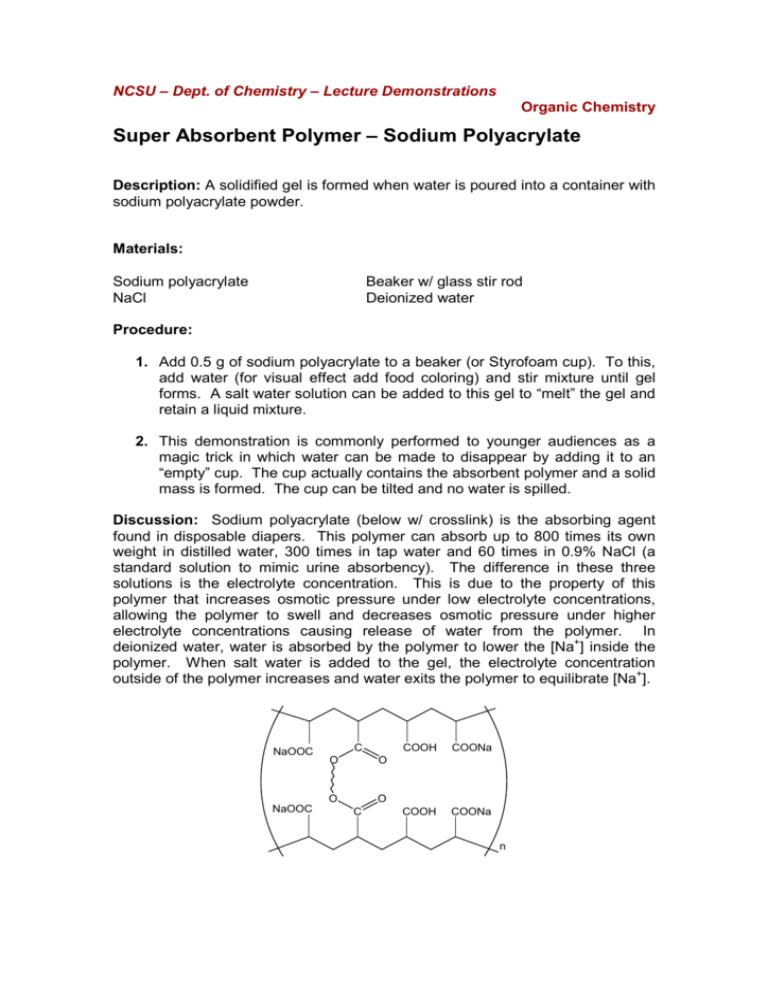
NCSU – Dept. of Chemistry – Lecture Demonstrations Organic Chemistry Super Absorbent Polymer – Sodium Polyacrylate Description: A solidified gel is formed when water is poured into a container with sodium polyacrylate powder. Materials: Sodium polyacrylate NaCl Beaker w/ glass stir rod Deionized water Procedure: 1. Add 0.5 g of sodium polyacrylate to a beaker (or Styrofoam cup). To this, add water (for visual effect add food coloring) and stir mixture until gel forms. A salt water solution can be added to this gel to “melt” the gel and retain a liquid mixture. 2. This demonstration is commonly performed to younger audiences as a magic trick in which water can be made to disappear by adding it to an “empty” cup. The cup actually contains the absorbent polymer and a solid mass is formed. The cup can be tilted and no water is spilled. Discussion: Sodium polyacrylate (below w/ crosslink) is the absorbing agent found in disposable diapers. This polymer can absorb up to 800 times its own weight in distilled water, 300 times in tap water and 60 times in 0.9% NaCl (a standard solution to mimic urine absorbency). The difference in these three solutions is the electrolyte concentration. This is due to the property of this polymer that increases osmotic pressure under low electrolyte concentrations, allowing the polymer to swell and decreases osmotic pressure under higher electrolyte concentrations causing release of water from the polymer. In deionized water, water is absorbed by the polymer to lower the [Na+] inside the polymer. When salt water is added to the gel, the electrolyte concentration outside of the polymer increases and water exits the polymer to equilibrate [Na+]. NaOOC C O O NaOOC COOH COONa COOH COONa O O C n NCSU – Dept. of Chemistry – Lecture Demonstrations Organic Chemistry Safety: Inhalation of the powdered polymer may be hazardous. Disposal: Place solid contents in a normal waste container. References: Shakhashiri, B. Z. In Chemical Demonstrations: A Handbook for Teachers of Chemistry; The University of Wisconsin Press: 1989; Vol. 3, p 368-371. Yaung, J.; Chen, Y. J. Chem. Educ. 2009, 86, 347. Criswell, B. J. Chem. Educ. 2006, 83, 574 and 576A. Buchholz, F. L. J. Chem. Educ. 1996, 73, 512. Video: http://www.youtube.com/watch?v=2errdUFTo58 http://www.youtube.com/watch?v=Vais8pL0w8U