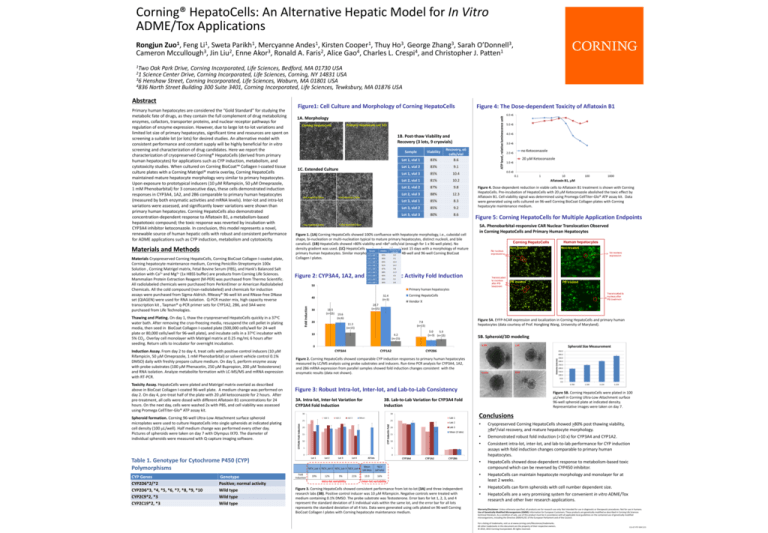
Corning® HepatoCells: An Alternative Hepatic Model for In Vitro ADME/Tox Applications
Rongjun Zuo1, Feng Li1, Sweta Parikh1, Mercyanne Andes1, Kirsten Cooper1, Thuy Ho3, George Zhang3, Sarah O’Donnell3, Cameron Mccullough3, Jin Liu2, Enne Akor3, Ronald A. Faris2, Alice Gao4, Charles L. Crespi4, and Christopher J. Patten1
1Two Oak Park Drive, Corning Incorporated, Life Sciences, Bedford, MA 01730 USA
21 Science Center Drive, Corning Incorporated, Life Sciences, Corning, NY 14831 USA
36
Henshaw Street, Corning Incorporated, Life Sciences, Woburn, MA 01801 USA
4836 North Street Building 300 Suite 3401, Corning Incorporated, Life Sciences, Tewksbury, MA 01876 USA
Abstract
Primary human hepatocytes are considered the “Gold Standard” for studying the metabolic fate of drugs, as they contain the full complement of drug metabolizing enzymes, cofactors, transporter proteins, and nuclear receptor pathways for regulation of enzyme expression. However, due to large lot‐to‐lot variations and limited lot size of primary hepatocytes, significant time and resources are spent on screening a suitable lot (or lots) for desired studies. An alternative model with consistent performance and constant supply will be highly beneficial for in vitro screening and characterization of drug candidates. Here we report the characterization of cryopreserved Corning® HepatoCells (derived from primary human hepatocytes) for applications such as CYP induction, metabolism, and cytotoxicity studies. When cultured on Corning BioCoat™ Collagen I‐coated tissue culture plates with a Corning Matrigel® matrix overlay, Corning HepatoCells maintained mature hepatocyte morphology very similar to primary hepatocytes. Upon exposure to prototypical inducers (10 M Rifampicin, 50 M Omeprazole, 1 mM Phenobarbital) for 3 consecutive days, these cells demonstrated induction responses in CYP3A4, 1A2, and 2B6 comparable to primary human hepatocytes (measured by both enzymatic activities and mRNA levels). Inter‐lot and intra‐lot variations were assessed, and significantly lower variations were shown than primary human hepatocytes. Corning HepatoCells also demonstrated concentration‐dependent response to Aflatoxin B1, a metabolism‐based hepatotoxic compound; the toxic response was reverted by incubation with CYP3A4 inhibitor ketoconazole. In conclusion, this model represents a novel, renewable source of human hepatic cells with robust and consistent performance for ADME applications such as CYP induction, metabolism and cytotoxicity.
Materials and Methods
Materials Cryopreserved Corning HepatoCells, Corning BioCoat Collagen I‐coated plate, Corning hepatocyte maintenance medium, Corning Penicillin‐Streptomycin 100x Solution , Corning Matrigel matrix, Fetal Bovine Serum (FBS), and Hank’s Balanced Salt solution with Ca2+ and Mg2+ (1x HBSS buffer) are products from Corning Life Sciences. Mammalian Protein Extraction Reagent (M‐PER) was purchased from Thermo Scientific. All radiolabeled chemicals were purchased from PerkinElmer or American Radiolabeled Chemicals. All the cold compound (non‐radiolabeled) and chemicals for induction assays were purchased from Sigma‐Aldrich. RNeasy® 96‐well kit and RNase‐free DNase set (QIAGEN) were used for RNA isolation. Q‐PCR master mix, high capacity reverse transcription kit , Taqman® q‐PCR primer sets for CYP1A2, 2B6, and 3A4 were purchased from Life Technologies.
Figure1: Cell Culture and Morphology of Corning HepatoCells B
1A. Morphology
Primary Hepatocyte Lot 321
Corning HepatoCells
D
1B. Post‐thaw Viability and Recovery (3 lots, 9 cryovials)
E
1C. Extended Culture Sample
Viability
Lot 1, vial 1
83%
Recovery, e6 cells/vial
8.6
Lot 1, vial 2
83%
9.1
Lot 1, vial 3
85%
10.4
Lot 2, vial 1
81%
10.2
Lot 2, vial 2
87%
9.8
Lot 2, vial 3
88%
12.3
Lot 3, vial 1
85%
8.3
Lot 3, vial 2
85%
9.2
Lot 3, vial 3
80%
8.6
Figure 1. (1A) Corning HepatoCells showed 100% confluence with hepatocyte morphology, i.e., cuboidal cell shape, bi‐nucleation or multi‐nucleation typical to mature primary hepatocytes, distinct nucleoli, and bile canaliculi. (1B) HepatoCells showed >80% viability and >8e6 cells/vial (enough for 1 x 96‐well plate). No density gradient was used. (1C) HepatoCells can be cultured for at least 15 days with a morphology of mature primary human hepatocytes. Similar morphology was observed on 48‐well and 96‐well Corning BioCoat Collagen I plates. Toxicity Assay. HepatoCells were plated and Matrigel matrix overlaid as described above in BioCoat Collagen I‐coated 96‐well plate. A medium change was performed on day 2. On day 4, pre‐treat half of the plate with 20 M ketoconazole for 2 hours. After pre‐treatment, all cells were dosed with different Aflatoxin B1 concentrations for 24 hours. On the next day, cells were washed 2x with PBS, and cell viability was assessed using Promega CellTiter‐Glo® ATP assay kit. 5A. Phenobarbital‐responsive CAR Nuclear Translocation Observed in Corning HepatoCells and Primary Human Hepatocytes 5B. Spheroid/3D modeling
Figure 2. Corning HepatoCells showed comparable CYP induction responses to primary human hepatocytes measured by LC/MS analysis using probe substrates and inducers. Run‐time PCR analysis for CYP3A4, 1A2, and 2B6 mRNA expression from parallel samples showed fold induction changes consistent with the enzymatic results (data not shown).
6.25K
1.6K
3.125K
0.8K
Figure 3: Robust Intra‐lot, Inter‐lot, and Lab‐to‐Lab Consistency
3A. Intra‐lot, Inter‐lot Variation for CYP3A4 Fold Induction
Figure 5B. Corning HepatoCells were plated in 100 L/well in Corning Ultra‐Low Attachment surface 96‐well spheroid plate at indicated density. Representative images were taken on day 7.
3B. Lab‐to‐Lab Variation for CYP3A4 Fold Induction
Conclusions
Table 1. Genotype for Cytochrome P450 (CYP) Polymorphisms Genotype
Positive; normal activity
Wild type
Wild type
Wild type
Figure 5: Corning HepatoCells for Multiple Application Endpoints
Figure 5A. EYFP‐hCAR expression and localization in Corning HepatoCells and primary human hepatocytes (data courtesy of Prof. Hongbing Wang, University of Maryland).
Spheroid formation. Corning 96‐well Ultra‐Low Attachment surface spheroid microplates were used to culture HepatoCells into single spheroids at indicated plating cell density (100 L/well). Half medium change was performed every other day. Pictures of spheroids were taken on day 7 with Olympus IX70. The diameter of individual spheroids were measured with Q‐capture imaging software.
CYP Genes
CYP2D6*2/*2
CYP2D6*3, *4, *5, *6, *7, *8, *9, *10
CYP2C9*2, *3
CYP2C19*2, *3
Figure 4. Dose‐dependent reduction in viable cells to Aflatoxin B1 treatment is shown with Corning HepatoCells. Pre‐incubation of HepatoCells with 20 M Ketoconazole abolished the toxic effect by Aflatoxin B1. Cell viability signal was determined using Promega CellTiter‐Glo® ATP assay kit. Data were generated using cells cultured on 96‐well Corning BioCoat Collagen plates with Corning hepatocyte maintenance medium.
Figure 2: CYP3A4, 1A2, and 2B6 Enzymatic Activity Fold Induction Thawing and Plating. On day 1, thaw the cryopreserved HepatoCells quickly in a 37oC water bath. After removing the cryo‐freezing media, resuspend the cell pellet in plating media, then seed in BioCoat Collagen I‐coated plate (500,000 cells/well for 24‐well plate or 80,000 cells/well for 96‐well plate), and incubate cells in a 37oC incubator with 5% CO2. Overlay cell monolayer with Matrigel matrix at 0.25 mg/mL 6 hours after seeding. Return cells to incubator for overnight incubation.
Induction Assay. From day 2 to day 4, treat cells with positive control inducers (10 M Rifampicin, 50 M Omeprazole, 1 mM Phenobarbital) or solvent vehicle control 0.1% DMSO) daily with freshly prepare culture medium. On day 5, perform enzyme assay with probe substrates (100 M Phenacetin, 250 M Bupropion, 200 M Testosterone) and RNA isolation. Analyze metabolite formation with LC‐MS/MS and mRNA expression with RT‐PCR.
Figure 4: The Dose‐dependent Toxicity of Aflatoxin B1 •
Cryopreserved Corning HepatoCells showed >80% post thawing viability, >8e6/vial recovery, and mature hepatocyte morphology.
•
•
Demonstrated robust fold induction (>10 x) for CYP3A4 and CYP1A2.
Consistent intra‐lot, inter‐lot, and lab‐to‐lab performance for CYP induction assays with fold induction changes comparable to primary human hepatocytes.
HepatoCells showed dose‐dependent response to metabolism‐based toxic compound which can be reversed by CYP450 inhibitor.
•
Mean %CV_Lot 1 %CV_lot 2 %CV_Lot 3 %CV_Lot 4
(all lots)
Fold Induction
19%
12%
9%
Intra‐lot variability
21%
19.9
%CV (all lots)
16%
•
HepatoCells can maintain hepatocyte morphology and monolayer for at least 2 weeks.
•
•
HepatoCells can form spheroids with cell number dependent size.
HepatoCells are a very promising system for convenient in vitro ADME/Tox research and other liver research applications.
Inter‐lot variability
Figure 3. Corning HepatoCells showed consistent performance from lot‐to‐lot (3A) and three independent research labs (3B). Positive control inducer was 10 M Rifampicin. Negative controls were treated with medium containing 0.1% DMSO. The probe substrate was Testosterone. Error bars for lot 1, 2, 3, and 4 represent the standard deviation of 3 individual vials within the same lot, and the error bar for all lots represents the standard deviation of all 4 lots. Data were generated using cells plated on 96‐well Corning BioCoat Collagen I plates with Corning hepatocyte maintenance medium. Warranty/Disclaimer: Unless otherwise specified, all products are for research use only. Not intended for use in diagnostic or therapeutic procedures. Not for use in humans. Use of Genetically Modified Microorganisms (GMM): Information for European Customers: These products are genetically modified as described in Corning Life Sciences technical literature. As a condition of sale, use of this product must be in accordance with all applicable local guidelines on the contained use of genetically modified microorganisms, including the Directive 2009/41/EC of the European Parliament and of the Council.
For a listing of trademarks, visit us at www.corning.com/lifesciences/trademarks.
All other trademarks in this document are the property of their respective owners.
© 2014, 2015 Corning Incorporated. All rights reserved.
CLS‐GT‐PST‐004 5/15
© 2015 Corning Incorporated
.
.
.