Sensors and Actuators B 91 (2003) 316–319
Mass-sensitive detection of cells, viruses and enzymes
with artificial receptors
Oliver Hayden, Roland Bindeus, Claudia Haderspöck, Karl-Jürgen Mann,
Barbara Wirl, Franz L. Dickert*
Institute of Analytical Chemistry, Vienna University, Waehringerstrasse 38, A-1090 Vienna, Austria
Abstract
Synthetic polymer receptors for the online monitoring of bioanalytes are formed directly onto quartz crystal microbalances using surface
imprinting techniques. The molded materials are capable of enriching whole cells, viruses and enzymes on the sensor layer surface. Enzyme
imprinted polymer layers are also effective as nucleation site for the induction of protein crystallization. Differential measurements are done
with a single piezocrystal having two screen-printed gold electrodes for a sensitive and a reference channel.
# 2003 Elsevier Science B.V. All rights reserved.
Keywords: Quartz crystal microbalance; Molecular imprinting; Cell; Virus; Enzyme; Crystallization
1. Introduction
Self-organized materials with specific properties are a
matter of intense research in various research fields. One
major application area of those so-called smart or advanced
materials is chemical sensing. Templating techniques, such
as molecular imprinting (MIP; molecularly imprinted polymers), are a promising approach to form receptor sites in the
bulk [1,2] for e.g. small organic molecules and on the surface
[3]. We have shown in recent publications that yeast
imprinted polyurethane sensor layers in combination with
microbalances can exclusively detect yeast cells without
cross-selectivities to bacteria [4]. The concept of cell
imprinting is based on a stamping technique, where templating cells are pressed with little force into a polymerizing
sensor layer. A honeycomb patterned surface remains after
the polymerization, which recognizes cells depending on
geometrical fit and chemical interaction [5].
The detection of viruses and bacteria is of great interest,
since both groups of analytes are a matter of big concern
regarding the contamination of water or their hazardous impact
as biological weapons. However, virus and bacteria detection
aregenerallytime-consumingtasks[6–8].Furthermore,weare
interested in expanding the concept of bioimprinting to
enzymes and to mammalian cells, which is crucial due to their
lower mechanical stability compared to microorganisms. For
*
Corresponding author. Tel.: þ43-1-4277-52317;
fax: þ43-1-4277-9523.
E-mail address: franz.dickert@univie.ac.at (F.L. Dickert).
chemical sensors the enzyme is still too large for bulk imprinting and surface imprinting has to be done again. Here, we
present the versatile concept of surface imprinted sensor layers
with stamping techniques, which extends the chemical sensing
applications of MIPs in aqueous phases to these nano- and
micrometer sized groups of bio-analytes.
2. Experimental
2.1. Dual QCMs and oscillator measurements
Gold electrodes were screen printed onto AT-cut 10 MHz
quartz blanks and subsequently burned in at 400 8C. Electrodes facing the aqueous phase have 50% larger diameters
than the electrodes oriented to the gas phase [9]. QCMs were
mounted into thermostated fluid cells. Electrodes were
connected to self-made oscillator circuits by soldering silver
wires to the QCM rims of coppered gold electrode contacts.
Electrodes in contact with the liquid are grounded as well as
the metallic liquid cell, which forms an faradaic cage. The
frequencies are recorded with a HP 53131A and data
acquisition is done via a HP-IB bus to a computer.
2.2. Monomers and analytes
Methacrylic acid was distilled to remove the stabilizer.
Radical inhibitors in styrene and divinylbenzene were
extracted with 2 M KOH and the monomers dried over
NaSO4. Other reagents were used as received. Blood sam-
0925-4005/03/$ – see front matter # 2003 Elsevier Science B.V. All rights reserved.
doi:10.1016/S0925-4005(03)00093-5
O. Hayden et al. / Sensors and Actuators B 91 (2003) 316–319
317
ples were purchased from the Austrian Red Cross. Tobacco
mosaic virus (TMV) has been gained from systemically
infected Nicotiana tabacum and purified according to the
literature [10]. Highly purified chicken egg white lysozyme
was purchased from Sigma.
2.3. Lysozyme imprinted polymer
A 1:106 diluted monomer solution in tetrahydrofurane
(THF) composed of a monomer mixture of styrene:divinylbenzene:methacrylic acid in 25:50:25 (w/w) with 1% AIBN
was spin coated at 2000 rpm onto the QCMs. The sensor
layers formed were transparent and defect free. Lysozyme
was crystallized on quartz glass from a 20 mg/ml solution in
a 65 mM sodium acetate buffer solution (pH 4.5, 5% NaCl)
[11]. The film of protein crystals was used for the stamping
of the sensor layer. Stamps are pressed on the QCMs during
the UV polymerization. Templating enzymes are washed-off
with 10 mM SDS. The crystallization on nucleating MIPs
was observed in equal conditions with a sitting drop method
from 5 mg/ml lysozyme according to the literature [12].
2.4. TMV imprinted polymer
Undiluted monomer solutions (cf. lysozyme MIP) were
prepolymerized 5 min at 70 8C and the solution spin cast on
the QCMs. TMV stamps are formed on an amylose layer on
quartz. The amylose is spin coated at 2500 rpm from a hot
aqueous solution onto the stamp. The virus suspension is
dropped on the amylose layer and dried at 35 8C for 15 min.
Stamps are again clipped on the QCMs during UV polymerization. Both templates and stamps are removed in hot
water and residual adhered TMV are washed-off with 2%
SDS. We experienced a steadily decreasing quality of the
TMV, when stored at temperatures around 20 8C (controlled with AFM). A good quality was maintained with a
storage temperature of 80 8C.
2.5. Erythrocyte MIP
Red blood cell ghosts were prepared from Aþ whole
blood according to the literature [13]. Monomer solutions
from THF with 47.8% bisphenol A, 12% phloroglucinol and
40.2% 4,40 -diisocyanato diphenlymethane (30% tri-isocyanato diphenlymethane) were drop coated on QCMs. Ghosts
were drop coated on glass slides and the stamps again
pressed on the coated QCMs. Templating cells were washed
with PBS buffer after polyaddition overnight.
3. Results
3.1. Compensated mass-sensitive measurements
Compensation of temperature effects is necessary, particularly in liquid environments, where viscosity changes will
Fig. 1. (A) Screen-printed dual microbalance on a single piezocrystal
(15.5 mm diameter). (B) Compensated dual QCM measurement with a
flow rate of 5 ml/min.
influence the measurements. However, different quartz
blanks often show slight differences in the cutting angles
although being from the same batch, which results in
different temperature dependencies. Fig. 1A shows a picture
of dual QCM with two electrodes on a single piezocrystal. In
this way, effective compensation of temperature and thus
viscosity fluctuations can be done, since the cutting angle for
both microbalances is identical. The frequency changes due
to temperature is shown in Fig. 1B. Temperature increase of
48 results in a positive frequency shift of 100 Hz. Both
electrodes (sensitive and reference channel) are affected in
the same manner. The compensated sensor response shows
the effective elimination of this influence with a flow rate of
5 ml/min (cell volume 150 ml). Compensation without time
delay can be achieved using analogue mixers in the oscillator circuits.
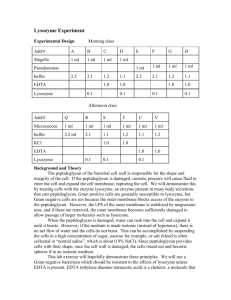
3.2. Detection of lysozyme and lysozyme crystallization
The detection of enzymes is of great interest, e.g. in the
detergent industry. Online measurements of protein concentrations with MIPs in aqueous solutions are feasible but
problems can occur with unspecific protein adsorption.
The imprinted polymer used for the detection of viruses
and enzymes is moderately hydrophobic and tends to form
intramolecular dimerization of the carboxylic functionalities.
At the sites where templates are in contact with the polymerizing film, hydrogen bonding between the template and
the crosslinked polymer might occur. Fig. 2A shows the
sensor response of the lysozyme imprinted polymer, which
exclusively shows a gravimetric response on the imprinted
318
O. Hayden et al. / Sensors and Actuators B 91 (2003) 316–319
Fig. 2. (A) Sensor effect to 4 mg/ml lysozyme in PBS buffer and a flow rate of 250 ml/min. The non-imprinted reference shows no effect. (B) AFM image of
lysozyme crystal. Insert shows a picture of lysozyme crystallized on the imprinted polymer surface from a solution of 5 mg/ml at 20 8C. No crystallization
occurred on non-imprinted polymers or glass.
channel. The reference shows no sensor effect at all, although
lysozyme tends to adsorb on hydrophobic surfaces [14].
The imprinted polymer capable of recognizing the
enzyme also showed nucleating behavior for the crystallization of lysozyme. Although, in this case we used crystals
to imprint the polymer surface, polymers imprinted with
non-crystalline enzymes should give a similar effect. In
Fig. 2B lysozyme crystals on the stamp are shown (contact
mode AFM). The insert is a photo of tetragonal crystals [15]
growing on an imprinted polymer surface from a lysozyme
solution of 5 mg/ml (20 8C). The first lysozyme crystallization can be observed after one day on the enzyme MIP.
The picture was taken after 4 days of crystallization. No
crystallization can be observed on either glass nor on nonimprinted polymer with equal enzyme concentrations and
same crystallization conditions. Crystallization was carried
out at 0.1 M sodium acetate with 5% NaCl (pH 4.6) using a
sitting drop vapor diffusion method at 20 8C [16]. We
suppose molecular imprinting offers an elegant way to
reduce the induction time and the amount of protein concentration necessary for the crystal nucleation [17].
3.3. Plant virus sensitivity
The AFM image in Fig. 3A shows TMV rods, which self
organize similiar to liquid crystals. A sensor response to
10 mg/ml TMV in PBS buffer with a flow rate of 1 ml/min
can be seen in Fig. 3B and is reported for the first time. A
reversible interaction of the viruses with the surface is
achieved with the surface imprinting approach. The virus
is washed-off with PBS buffer. No sensor effect was
observed on the reference channel.
3.4. Chemical sensing of erythrocytes
Polyurethane is a very versatile material for chemically
sensitive layers. Compared to other polymers, it shows good
adhesion qualities to the microbalance surface and the
Fig. 3. (A) AFM tapping mode image showing single TMV rods on mica. (B) Sensor responses of imprinted and non-imprinted channel to 10 mg/ml TMV
with a flow rate of 1 ml/min in PBS buffer.
O. Hayden et al. / Sensors and Actuators B 91 (2003) 316–319
319
Acknowledgements
This work was supported by FWF (P15512).
References
Fig. 4. Sensor effect to 106 erythrocytes/ml in PBS buffer (flow rate 5 ml/
min). Red cells are washed-off with PBS.
polyaddition reaction allows an easier layer formation than
using radical polymerization conditions. However, the covalent embedding of bio-templates through reactive isocyanato
functionalities has to be suppressed. We use an excess of
phenolic functionalities and the prepolymerized state before
coating to minimize the amount of reactive groups on the
polymer surface. This approach was successfully applied for
the selective detection of yeasts. Erythrocyte ghosts had to
be prepared as templates, since native erythrocytes were
partially disrupted during the stamping process. Escaping
intracellular plasma interfered with the imprinting process.
The ghosts were useful templates and a sensor response to
106 cells/ml is shown in Fig. 4. The compensated measurement shows the erythrocyte detection with a flow rate of
5 ml/min in PBS at 20 8C. Adhered cells were washed-off
with a PBS washing step. The sensitive channel showed 2.5
times more effect than the non-imprinted reference channel.
4. Discussion
Molecular imprinting proved to be a versatile technique to
generate chemically sensitive layers for small organic molecules or ions [18]. Surface imprinting is also successfully
applicable to form fingerprints from nanometer or micrometer
sized templates. This concept to detect new groups of bioanalytes with synthetic receptors promises to be highly effective as being already reported with yeasts. Enzymes and
viruses can be effectively enriched on imprinted layers,
whereas the non-imprinted layers show minor or no unspecific
effects. Even whole mammalian cells, such as erythrocytes,
are showing to be affine to their fingerprints. In combination
with dual microbalances, a robust and compensated sensing
method is obtained for size-independent analyte detection.
The enzyme imprinted materials are suitable for crystallization. The ultimate goal of this strategy would be a
nucleation which can be achieved solely by imprinting with
a non-crystalline protein. Future work will improve the
templating techniques and the layer design for the upcoming
selectivity studies.
[1] F.L. Dickert, S. Thierer, Molecularly imprinted polymeres for
optochemical sensors, Adv. Mater. 8 (1996) 987–990.
[2] F.L. Dickert, H. Besenböck, M. Tortschanoff, Molecular imprinting
through van der Waals interactions: fluorescence detection of PAHs
in water, Adv. Mater. 10 (1998) 149–151.
[3] F.L. Dickert, O. Hayden, K.P. Halikias, Synthetic receptors as sensor
coatings for molecules and living cells, Analyst 126 (2001) 766–771.
[4] O. Hayden, F.L. Dickert, Selective microorganism detection with cell
surface imprinted polymers, Adv. Mater. 12 (2001) 311–313.
[5] F.L. Dickert, O. Hayden, Bioimprinting of polymers, Anal. Chem. 74
(2002) 1302–1306.
[6] E. de Boer, R.R. Beumer, Methodology for detection and typing of
foodborne microorganisms, Int. J. Food Microbiol. 50 (1999) 119–130.
[7] D. Ivnitski, I. Abdel-Hamid, P. Atanasov, E. Wilkins, Biosensors for
detection of pathogenic bacteria, Biosens. Bioelectron. 14 (1999)
599–624.
[8] M. Koopmans, C.-H. von Bonsdorff, J. Vinjé, D. de Medici, S. Monroe,
Foodborne viruses, FEMS Microbiol. Rev. 26 (2002) 187–205.
[9] F.L. Dickert, K. Halikias, O. Hayden, L. Ping, R. Sikorski, Sensors
based on fingerprints of neutral and ionic analytes in polymeric
materials, Sens. Actuators B 76 (2001) 295–298.
[10] H. Fraenkel-Conrat, R.C. Williams, Reconstruction of active tobacoo
mosaic virus from ist inactive protein and nucleic acid components,
Proc. Natl. Acad. Sci. USA 41 (1955) 690–698.
[11] L. Rong, T. Yamane, N. Niimura, Measurement and control of the
crystal growth rate of tetragonal hen egg-white lysozyme image with
an atomic force microscope, J. Crystal Growth 217 (2000) 161–169.
[12] J.P. Sumida, E.L. Forsythe, M.L. Pusey, Preparation and preliminary
characterization of crystallizing fluorescent derivatives of chicken
egg white lysozyme, J. Crystal Growth 232 (2001) 308–316.
[13] J.T. Dodge, C. Mitchell, D.J. Hanahan, The preparation and chemical
characteristics of hemoglobin free ghost of human erythocyte, Action
Biochem. Biophys. 100 (1963) 119–130.
[14] J. Su, R.J. Green, Y. Wang, E.F. Murphy, J.R. Lu, R. Ivkov, S.K.
Satija, Adsorption of lysozyme onto the silicon oxide surface
chemically grafted with a monolayer of pentadecyl-1-ol, Langmuir
16 (2000) 4999–5007.
[15] L. Rong, H. Komatsu, S. Yoda, Control of heterogenous nucleation
of lysozyme crystals by using poly-L-lysine modified substrate, J.
Crystal Growth 235 (2002) 489–493.
[16] J.P. Sumida, E.L. Forsythe, M.L. Pusey, Preparation and preliminary
characterization of crystallizing fluorescent derivatives of chicken
egg white lysozyme, J. Crystal Growth 232 (2001) 308–316.
[17] S. Fermani, G. Falini, M. Minnucci, A. Ripamonti, Protein crystallization on polymeric film surfaces, J. Crystal Growth 224 (2001)
327–334.
[18] K. Haupt, K. Mosbach, Molecularly imprinted polymers and their
use in biomimetic sensors, Chem. Rev. 100 (2000) 2495–2504.
Biographies
Oliver Hayden is a biochemist and received his PhD from Vienna
University. He is working in the group of F.L. Dickert and his main fields
of interests are artificial bioanalyte recognition and AFM techniques. R.
Bindeus (PhD candidate), C. Haderspöck (PhD candidate), K.J. Mann and
B. Wirl are members of F.L. Dickert’s group.
Franz L. Dickert studied chemistry at the University of Erlangen, Germany
and received his PhD in 1970. He was appointed as Professor of Physical
Chemistry in 1980. Since 1994 he holds a chair of Analytical Chemistry at
Vienna University. His activities are focused on developing chemical
sensors, especially on smart, sensitive materials.