Density Unit Study Guide
advertisement

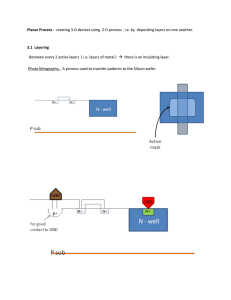
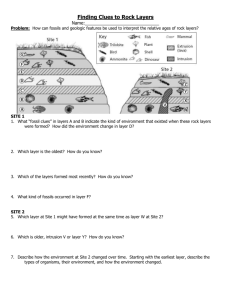
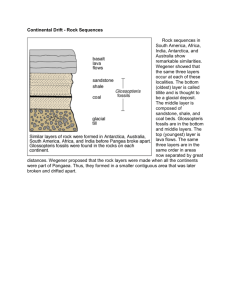
Density Unit Study Guide Note: This is not a “cover all” study guide. There may be some questions on the test that we covered in class, but I do not ask you here. This is just to help you get an idea of what you need to know for the test. Answer these questions on a separate piece of paper. 1. 2. 3. 4. 5. 6. 7. 8. 9. What is equation for density? What is density? Put this into words that make sense to you. Draw pictures to help you. Draw and label the layers of the Earth from the core to space. Give details about each layer you drew in question number 3. Explain how you would find the density of a gas. How do you find the volume of an irregular shaped object? How do you find the volume of a rectangular shaped object? How do you find the volume of a liquid? Solve the following: a. What is the density of an object whose mass is 35 g and volume is 60 cc. b. What is the volume of an object whose length is 3 cm, width is 4 cm, and height is 4 cm. c. What is the density of an object whose mass is 10 g and volume is 15 mL. d. What is the density of an object whose mass is 3 g and length is 1 cm, width is 1 cm and height is 2 cm. e. What is the mass of an object whose density is 5 g/cc and volume is 2 cc. 10. How do scientists know about Earth’s interior? 11. How would a mixture of sediment sort if all of the particles were of equal densities but of different sizes? 12. How would a mixture of sediment sort if all of the particles were of equal size but of different densities? 13. What affects how layers form in streambeds? 14. What affects how layers form in the air? 15. What affects how layers form in soil and rocks? 16. Why does our Earth’s interior have layers? 17. The density of water is 1 g/mL, the density of mahogany wood is .5 g/cc, the density of an iron nail is 7.8 g/cc, the density of corn syrup is 1.3 g/cL and the density of oil is .9 g/mL. Draw a diagram showing how these items would sort if placed into a large beaker. Be sure to label your items.