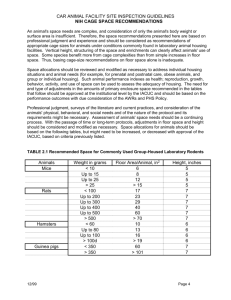
Chapter 4 - Office of Research - University of California, Irvine
advertisement

Program Description Animal Care and Use Program File number 000238 University of California Irvine Office of Research 5171 California Avenue, Suite 150 Irvine, CA 92697-7600 For AAALAC International April 1, 2012 2/11 Table of Contents Section 1. Introduction ................................................................................................................. 1 Section 2. Description .................................................................................................................. 5 I. Animal Care and Use Program ............................................................................................... 5 A. Program Management ......................................................................................................... 5 1. Program Management Responsibility ......................................................................... 5 a. The Institutional Official ............................................................................................. 5 b. The Attending Veterinarian ......................................................................................... 5 c. Collaborations ............................................................................................................. 6 2. Personnel Management ............................................................................................... 7 a. Training and Education ............................................................................................... 7 i. Veterinary and Other Professional Staff .............................................................. 7 ii. Animal Care Personnel ........................................................................................ 8 iii. The Research Team.............................................................................................. 9 b. Occupational Health and Safety of Personnel ........................................................... 12 i. Hazard Identification and Risk Assessment ...................................................... 13 ii. Facilities, Equipment and Monitoring ............................................................... 15 iii. Personnel Training ............................................................................................. 19 iv. Personal Hygiene ............................................................................................... 20 v. Animal Experimentation Involving Hazards ..................................................... 21 vi. Personal Protection ............................................................................................ 26 vii. Medical Evaluation and Preventive Medicine for Personnel ............................. 28 c. Investigating and Reporting Animal Welfare Concerns ........................................... 30 B. Program Oversight ............................................................................................................ 31 1. The Role of the IACUC/OB ...................................................................................... 31 a. IACUC/OB Composition and Function .................................................................... 31 b. Protocol Review ........................................................................................................ 32 c. Special Considerations for IACUC/OB Review ....................................................... 34 i. Experimental and Humane Endpoints ............................................................... 34 ii. Unexpected Outcomes that Affect Animal Well-being ..................................... 35 iii. Physical Restraint............................................................................................... 35 iv. Multiple Survival Surgical Procedures .............................................................. 36 v. Food and Fluid Regulation................................................................................. 37 vi. Use of Non-Pharmaceutical-Grade Drugs and Other Substances...................... 39 vii. Field Investigations ............................................................................................ 39 viii. Agricultural Animals ......................................................................................... 40 ix. Animal Reuse ..................................................................................................... 40 2. Post-Approval Monitoring ........................................................................................ 40 II. Animal Environment, Housing and Management ................................................................ 42 A. Animal Environment ......................................................................................................... 42 1. Temperature and Humidity ....................................................................................... 42 2. Ventilation and Air Quality ....................................................................................... 43 3. Life Support Systems for Aquatic Species ................................................................ 44 4. Noise and Vibration................................................................................................... 44 B. Animal Housing (All terrestrial, flighted, and aquatic species)........................................ 44 1. Primary Enclosures.................................................................................................... 44 2. Environmental Enrichment, Social and Behavioral Management ............................ 45 a. Enrichment ................................................................................................................ 45 i 2/11 b. Social Environment ................................................................................................... 46 c. Procedural Habituation and Training of Animals ..................................................... 47 d. Enrichment, Social and Behavioral Management Program Review ......................... 47 e. Sheltered or Outdoor Housing................................................................................... 47 f. Naturalistic Environments ......................................................................................... 48 C. Animal Facility Management ........................................................................................... 49 1. Husbandry ................................................................................................................. 49 a. Food ........................................................................................................................... 49 b. Drinking Water .......................................................................................................... 51 c. Bedding and Nesting Materials ................................................................................. 52 d. Miscellaneous Animal Care and Use Equipment ...................................................... 53 e. Sanitation ................................................................................................................... 53 f. Waste Disposal .......................................................................................................... 55 g. Pest Control ............................................................................................................... 56 h. Emergency, Weekend and Holiday Care .................................................................. 57 2. Population Management ............................................................................................ 58 a. Identification ............................................................................................................. 58 b. Record Keeping ......................................................................................................... 58 c. Breeding, Genetics and Nomenclature ...................................................................... 58 III. Veterinary Care ................................................................................................................. 59 A. Animal Procurement and Transportation .......................................................................... 59 1. Animal Procurement.................................................................................................. 59 2. Transportation of Animals......................................................................................... 60 B. Preventive Medicine ......................................................................................................... 60 1. Animal Biosecurity.................................................................................................... 61 2. Quarantine and Stabilization ..................................................................................... 61 3. Separation by Health Status and Species .................................................................. 63 4. Surveillance, Diagnosis, Treatment and Control of Disease ..................................... 63 C. Clinical Care and Management ......................................................................................... 65 1. Emergency Care ........................................................................................................ 65 2. Clinical Record keeping ............................................................................................ 65 3. Diagnostic Resources ................................................................................................ 66 4. Drug Storage and Control.......................................................................................... 66 D. Surgery .............................................................................................................................. 67 1. Pre-Surgical Planning ................................................................................................ 67 2. Surgical Facilities ...................................................................................................... 68 3. Surgical Procedures ................................................................................................... 70 4. Aseptic Technique ..................................................................................................... 70 5. Intraoperative Monitoring ......................................................................................... 71 6. Postoperative Care ..................................................................................................... 71 E. Pain and Distress ............................................................................................................... 72 F. Anesthesia and Analgesia ................................................................................................. 73 G. Euthanasia ......................................................................................................................... 76 IV. Physical Plant .................................................................................................................... 78 A. Location and Construction Guidelines.............................................................................. 78 B. Functional Areas and Operations ...................................................................................... 81 1. Heating, Ventilation, and Air-Conditioning (HVAC) ............................................... 81 2. Power and Lighting ................................................................................................... 82 3. System Malfunctions ................................................................................................. 84 ii 2/11 Storage Areas ............................................................................................................ 84 Facilities for Sanitizing Materials ............................................................................. 85 C. Special Facilities ............................................................................................................... 87 1. Specialized Types of Animal Housing ...................................................................... 88 2. Surgery ...................................................................................................................... 88 3. Other Specialized Animal Use Facilities................................................................... 89 4. Other Animal Support Facilities................................................................................ 89 D. Security and Access Control ............................................................................................. 89 4. 5. Appendices 1. 2. 3. 4. 5. 6. 7. 8. 9. 10. 11. 12. 13. Organizational Chart(s) Animal Usage Form A or B Summary of Animal Housing and Support Sites Line Drawings Medical Evaluation Form IACUC/OB Membership Roster Blank IACUC/OB Protocol Form IACUC/OB Minutes IACUC/OB Periodic Report Heating, Ventilation and Air Conditioning (HVAC) System Summary Aquatic Systems Summary Primary Enclosures and Animal Space Provisions Cleaning and Disinfection of the Micro- and Macro-Environment iii 2/11 Program Description Link to Instructions for Completing and Submitting the Program Description for the Institutional Animal Care and Use Program Section 1. Introduction A. State the name of the program unit and, if applicable, its parent organization. List all organizations (schools, centers, etc.) included within the program unit. The University of California, Irvine (UCI) has one animal care and use program (the “Animal Program”) which is centrally administered by University Laboratory Animal Resources (ULAR), the Institutional Animal Care and Use Committee (IACUC) and the Office of Research Administration (ORA) within the Office of Research (OR). The Animal Program covers research activities in the School of Biological Sciences (SBS), the School of Medicine (SOM), School of Engineering (SOE), School of Physical Sciences (SPS), Department of Pharmaceutical Sciences and all campus research centers and institutes. Animal Program research sites are located on the central campus (Irvine, CA) as well as the UCI Medical Center (Orange, CA). B. Give a brief overview of the institution, its purpose and how the animal care and use program relates to the mission of the institution. The University of California (UC) system is comprised of ten campuses throughout California and is one of the world’s largest and most renowned centers of higher education. The Irvine campus (UCI) opened in 1965 and has since become an academic and research institution of national and international stature, committed to the pursuit of quality research and transmission of knowledge. At the undergraduate and graduate levels, UCI provides students with access to faculty members who conduct research at the forefront of their field. The Animal Program has the dual responsibility of supporting the teaching and research missions of UCI, while ensuring the ethical care and use of all vertebrate animals used in research, testing, teaching and related purposes. University Laboratory Animal Resources (ULAR) functions primarily as the service arm of the Animal Program with responsibilities for veterinary care, animal husbandry, administrative support and animal facility housekeeping/maintenance. The Office of Research (OR) is UCI's primary institutional agent in administering federal, state, and private foundation regulations and strives to maintain an ethical environment for research. Policy guidance and regulatory oversight for the Animal Program are provided by Research Protections, a subunit of the Office of Research, and the Institutional Animal Care and Use Committee (IACUC). C. Note that AAALAC International’s three primary standards are the Guide for the Care and Use of Laboratory Animals (Guide), NRC, 2011; the Guide for the Care and Use of Agricultural Animals in Research and Teaching (Ag Guide), FASS 2010, and the European Convention for the Protection of Vertebrate Animals Used for Experimental 1 2/11 and Other Scientific Purposes, Council of Europe (ETS 123). Other regulations and guidelines used (U.S. Department of Agriculture (USDA), Public Health Service (PHS) Policy, Good Laboratory Practice (GLP), Canadian Council on Animal Care (CCAC), etc.) may also apply. Describe which of the three primary standards and other regulations and guidelines are used as standards for the institutional animal care and use program and how they are applied. For example, an academic institution in the United States with an Office of Laboratory Animal Welfare (OLAW) Assurance may use the standards of the Guide and PHS Policy for all animals, the Animal Welfare Act regulations for covered species, and the Ag Guide for agricultural animals used in agricultural research and teaching. In the European Union, the standards applied might be the Guide, ETS 123, Directive 2010/63, and any country-specific regulations. The Animal Program uses the Guide for the Care and Use of Laboratory Animals (Guide), NRC 2011 as the primary evaluation standard for its Animal Program, in addition to the Animal Welfare Act/Regulations and the Public Health Service Policy. These standards are applied to all vertebrate animal species, regardless of funding source. D. Describe the organization and include an organizational chart or charts (as an Appendix/Appendices) detailing the lines of authority from the Institutional Official to the Attending Veterinarian, the Institutional Animal Care and Use Committee/Oversight Body (IACUC/OB), and the personnel providing animal care. Please include the title, name (Note: For individuals whose information is publically available, provide the titles and names; for individuals whose information is not publically available, you may provide titles only.), and degree (if applicable) of each individual at the level of supervisor or above. Names of animal care staff below the title of supervisor need not be included, but the titles and number of animal care personnel under each supervisor should be included. If animal care responsibility is administratively decentralized, the organizational chart or charts must include all animal care programs, indicating the relationship between each administrative unit and personnel, the Attending Veterinarian, and the Institutional Official. The UC Board of Regents is appointed by the Governor of California and oversees the entire University system with full powers of organization and government. The Regents appoint the President of the University, as well as the Chancellors and laboratory Directors. Authority in academic matters is delegated by the Regents to the Academic Senate, which determines academic policy for the university as a whole. UC President Mark Yudof is the executive head of the ten-campus university system. Each of the campuses has a chancellor as its chief administrative officer. UCI’s Chancellor, Michael V. Drake, M.D., assumed his duties in 2005. Chancellor Drake is responsible for the organization and operation of the campus, including academic, student, and business affairs. The Chancellor's position has delegated Institutional Official (IO) authority with oversight and administrative duties of all aspects of the animal care and use program to the Vice Chancellor for Research, John C. Hemminger, Ph.D., who has further delegated the role of Institutional Official to Associate Vice Chancellor for Research James W. Hicks, Ph.D. Dr. Hicks assumed his duties as IO in 2011 and is responsible for compliance with applicable regulations set forth by all federal, state and local 2 2/11 agencies that regulate the ethical care and use of animals. He is assisted in the discharge of this responsibility by the IACUC, the Director of University Laboratory Animal Resources and Attending Veterinarian, and the Office of Research Administration. Additionally, institutional oversight and administrative duties for the department of Environmental Health and Safety (EH&S) have been delegated to the Vice Chancellor for Administrative and Business Services, Wendell Brase. The Department of Environmental Health and Safety (EH&S), under the direction of Dick Sun, ensures that UCI complies with applicable health, safety and environmental laws, regulations and requirements and that activities are conducted in a manner that protects students, faculty, staff, visitors, and the environment. See also Appendix 1 – Organizational Charts E. Identify the key institutional representatives (including, but not limited to, the Institutional Official; IACUC/OB Chairperson; Attending Veterinarian; animal program manager; individual(s) providing biosafety, chemical hazard, and radiation safety oversight; etc.); and individuals anticipated to participate in the site visit. James W. Hicks, Ph.D., Assoc Vice Chancellor for Research/Institutional Official Vincent Caiozzo, Ph.D., IACUC Chair Bruce Morgan, Assistant Vice Chancellor for Research Karen Allen, Director of Research Protections, Office of Research Administration Melanie Fabian, CPIA, IACUC Administrator, Office of Research Administration Jeffrey Goodwin, D.V.M., Ph.D., DACLAM, Director (Attending Veterinarian), ULAR Claire Lindsell, B.V.Sc., Ph.D., DACLAM, Associate Director, ULAR Roger Geertsema, D.V.M., DACPVM, DACLAM, Assistant Director, ULAR Jefferson Chau, B.S., CMAR, Animal Facility Operations Manager, ULAR Sheila Hedayati, M.P.H., Biosafety Officer, Environmental Health & Safety Lorena Andrade, B.S., Vivarium Biosafety Specialist, Environmental Health & Safety F. Briefly describe the major types of research, testing, and teaching programs involving animals and note the approximate number of principal investigators and protocols involving the use of animals. As mentioned in the instructions, please complete one of the animal use forms included with this outline or provide the information requested in a similar format as an appendix. The major areas of research and instruction that involve live animals at UCI include neuroanatomy and physiology; ophthalmology; viral research; animal models of brain diseases, spinal cord trauma and demyelination; minimallyinvasive robotic surgical training; virology; developmental and evolutionary biology; molecular biology and biochemistry; and basic and applied stem cell research. There are approximately 200 principal investigators currently involved in the use of live vertebrate animals, with approximately 400 active animal protocols. More than 95% of the vertebrate mammal research at UCI is performed in rodents. 3 2/11 G. Note the source(s) of research funding (grants, contracts, etc.) involving the use of animals. UCI receives extramural funding from both federal and non-federal agencies, including state and local funds, private for-profit and non-profit entities. H. List other units (divisions, institutes, areas, departments, colleges, etc.) of your organization that house and use animals that are not included in this Description. If any of these are contiguous, physically or operationally (e.g., same IACUC/OB, same animal care staff), with the applicant unit, describe the association. Explain why such units are not part of this program application. None I. Contract Facilities: If the institution contracts for animal care facilities or services for animals owned by the institution, the contractor and its AAALAC International accreditation status must be identified. If a contractor's animal care and use program is not accredited by AAALAC International, a brief description, following this Program Description outline, of the relevant contractor's programs and facilities must be provided. In addition, the species and approximate average number of animals housed in the contract facilities and the approximate distance between the institution's animal facility and the contract facility must be noted. Incorporation of the contractor program into the site visit schedule will be discussed with institutional representatives. If the institution does not contract for animal care facilities or services, so note. UCI does not currently contract for animal care facilities or services. J. Note other relevant background that will assist reviewers of this report. UCI holds Animal Welfare Assurance No. A3416-01 in accordance with PHS Policy for the Humane Care and Use of Laboratory Animals. The current Assurance became effective on February 15, 2012 and expires on February 28, 2016. 4 2/11 Section 2. Description I. Animal Care and Use Program A. Program Management 1. Program Management Responsibility [Guide, pp. 13-15] a. The Institutional Official [Guide p. 13-14] Describe how program needs are clearly and regularly communicated to the Institutional Official by the Attending Veterinarian, IACUC/OB, and others associated with the program. There are direct and open lines of communication between the IACUC and the Institutional Official (IO) and between the Veterinarian and the IO. There are regularly scheduled meetings of the Office of Research senior management group, including the Vice Chancellor for Research, Associate Vice Chancellor for Research/Institutional Official, Associate Vice Chancellor for Research Administration, Attending Veterinarian and Assistant Vice Chancellor for Research Administration. b. The Attending Veterinarian [Guide, p. 14] i. Describe the institutional arrangement for providing adequate veterinary care. For each veterinarian associated with the program (including private practitioners), provide the veterinarian's name(s), list responsibilities, and how the veterinarian is involved in monitoring the care and use of laboratory animals. If employed full-time by the institution, note the percentage of time devoted to supporting the animal care and use program of the institution. If employed part-time or as a consultant, note the frequency and duration of visits. Jeffrey Goodwin, D.V.M, Ph.D. serves on the IACUC, is the director of University Laboratory Animal Resources (ULAR) and is appointed by and reports to the Vice Chancellor for Research via both the Associate Vice Chancellor for Research/Institutional Official and the Associate Vice Chancellor for Research Administration. He supervises housing and husbandry and ensures that adequate veterinary care is provided to all animals used in research, teaching and testing. He is authorized to enter any place within the jurisdiction of UCI to conduct on-site inspections of animal facilities and animals to ensure that husbandry, sanitation practices and animal health and research techniques are in compliance with relevant federal, state, local and institutional requirements. The AV is authorized to halt any animal activity if the safety or welfare of an animal is at risk or if the work being performed on a project is not in accordance with the approved protocol. Dr. Goodwin also assists in coordinating the Animal Program, including the approval of animal facility renovations and 5 2/11 new construction; he is a full time employee of UCI and contributes 100% of his time to the animal care and use program. ii. List others (e.g., Principal Investigators, veterinarians serving as Principal Investigators, veterinary faculty/staff, technical staff, farm managers) who have a direct role in the provision of veterinary care and describe their responsibilities. An organizational chart depicting the reporting relationship between these individuals and the Attending Veterinarian should be included as an appendix. ULAR Associate Director Dr. Claire Lindsell and Assistant Director Dr. Roger Geertsema assist the Attending Veterinarian in the management of University Laboratory Animal Resources (ULAR). Drs. Goodwin, Lindsell and Geertsema, in collaboration with the Vivarium Operations Manager, establish the methods and procedures for the housing, feeding, care and use of all research animals housed at UCI facilities, including the design of new animal facilities and the renovation of existing facilities. ULAR is comprised of four functional areas: Operations and Finance, Vivarium Operations, Veterinary Services and the Transgenic Mouse Facility. Operations and Finance includes the centralized acquisition of research animals; Vivarium Operations provides oversight for the day-to-day husbandry and care of animals in UCI vivarium facilities. Veterinary services are provided by Drs. Goodwin, Lindsell and Geertsema with the assistance of three Animal Health Technicians. The Transgenic Mouse Facility (TMF) provides services for the production of genetically modified mice. c. Collaborations [Guide, p. 15] Describe processes for assigning animal care and use responsibility, animal ownership and IACUC/OB oversight responsibilities at off-site locations (i.e., collaborations). When proposed animal research performed by or under the direction of UCI personnel will be conducted entirely offsite (i.e., not at UCI facilities) at an OLAW-assured institution and the offsite location retains ownership of the animals, UCI may accept registration of the offsite entity’s IACUC approval in lieu of review and approval by the UCI IACUC. Registration of an offsite IACUC approval requires submission of the approved protocol and offsite entity’s approval letter to the UCI IACUC for administrative review. UCI reserves the right to limit, suspend or terminate the involvement of UCI personnel in studies approved by offsite entities. Offsite protocol registrations reviewed administratively are acknowledged at the next convened meeting of the IACUC and appended to the minutes. Protocol registration dates coincide with the offsite location’s approval period. All protocol modifications and annual renewals approved by the offsite entity’s 6 2/11 IACUC must be submitted to UCI’s IACUC in order to maintain the registration in good standing. In cases where a collaborator from another institution wishes to use UCI facilities and will retain ownership of the animals, a UCI protocol application must be reviewed and approved by the UCI IACUC which covers the activities to be performed on UCI property (e.g., whole-body irradiation, MRI scanning, etc.) A UCI faculty member eligible to serve as a Principal Investigator must be identified as responsible for the activities performed in UCI facilities. 2. Personnel Management a. Training and Education Describe how the IACUC/OB provides oversight and evaluates the effectiveness of training programs. Describe how training is documented. The IACUC routinely reassesses the training programs associated with animal care and use as part of the semi-annual Program Evaluation process. UCI’s program utilizes the Collaborative Institutional Training Initiative (CITI) program for basic training in animal use, as well as species- and procedure-specific training and training for new IACUC members. A nightly data-feed transmits completion information from CITI to the animal program database at UCI; the database has been programed to reject all individuals from inclusion on animal protocols until required training is completed. Classroom training/orientation is required by ULAR as a condition for animal-facility access. Records are maintained by ULAR and utilized in the process of obtaining a photo identification/vivarium access card. When questions regarding the training and/or expertise of research personnel arise during protocol review, the IACUC requests that the protocol be flagged for enhanced veterinary oversight and outreach in order to ensure that personnel are adequately trained to perform the approved procedures. The IACUC has also developed a training record template for use in laboratories to document training for all members of the laboratory staff in the specific procedures used in the lab. IACUC Facility Inspection teams have included the documentation of training as a topic for discussion with researchers during semi-annual Facility Inspections. The IACUC often requires retraining (e.g., repeating the CITI courses and/or classroom training) as a condition of resolution for regulatory non-compliance. i. Veterinary and Other Professional Staff [Guide, pp. 15-16] Provide name and credentials of veterinary and other professional staff, including the veterinary personnel listed above, and describe their qualifications, training, and continuing education. Please do not provide curriculum vitae of personnel. Jeffrey Goodwin, ULAR Director 7 2/11 • B.S. degree in Distributed Sciences from Iowa State University • D.V.M. degree from Iowa State University • Ph.D. degree in Anatomy from Iowa State University • Diplomate, ACLAM • Holds memberships in AALAS, AVMA, ASLAP • 18 years experience as a laboratory animal veterinarian Claire Lindsell, Associate Director • Bachelor of Veterinary Science from University of Sydney, Australia • M.S. from University of Saskatchewan, Canada • Ph.D. in Molecular Endocrinology from University of Saskatchewan, Canada • Diplomate, ACLAM • Holds memberships in AALAS, ASLAP • 15 years experience as veterinarian, 9 years in biomedical research Roger Geertsema, Assistant Director • B.S. degree in Veterinary Science from University of Minnesota • DVM degree from University of Minnesota • Diplomate, ACLAM and ACVPM, • Holds memberships in AALAS, AVMA, ASLAP • 28 years experience as veterinarian, including 12 years in private practice, 7 years in the military, and 9 years in biomedical research Andrea Ristig, Senior Animal Health Technician • Registered Laboratory Animal Technologist (RLATg) from AALAS • AA degree in Liberal Studies • 19 years laboratory animal experience Amilee Kaye, Animal Health Technician • Laboratory Animal Technologist (LATg) from AALAS • AS degree in Animal Science, certificate as Veterinary Technician • 6 years laboratory animal experience Sirinda Sriwanthana, Animal Health Technician • Laboratory Animal Technician (LAT) from AALAS • BS in animal science • 7 years laboratory animal experience ii. Animal Care Personnel [Guide, p. 16] Indicate the number of animal care personnel. 31 Summarize their training, certification level and type, experience, and continuing education opportunities provided. Training Program 8 2/11 ULAR utilizes a performance-based training program to develop and enhance the core and advanced skills of Animal and Cage Wash Technicians. This program was developed for new employees but all current staff have completed this training as well. Training for ULAR Animal Technicians is provided by an experienced Supervisor, Lead Technician and Veterinary staff and includes species-specific training modules (e.g. conventional mouse and rat husbandry module), and task-specific modules (e.g. cage wash operations module, animal health module). Training progresses at a rate dictated by the knowledge, understanding and abilities of the employee. This rate is assessed by the Trainer or Supervisor. Training modules may include: • Reading the applicable SOPs and asking questions • Observing the Trainer performing tasks • Performing tasks while being observed by the Trainer • Performing tasks alone with regular visits by the Trainer • Evaluation of competency by the Trainer • Documentation that the technician is qualified to perform the tasks included in the training module. In the initial training period, core training modules covering the major job duties will be completed. As operational needs arise, technicians are trained in additional duties. AALAS Certification Program There are currently 7 ALAT, 3 LAT, and 1 CMAR certified Animal Technicians on the ULAR staff (this includes Senior Animal Technicians and supervisory staff). The in-house AALAS certification training program consist of 2 weekly 1 hour (paid) training sessions for the ALAT and LAT training levels and is taught by ULAR Managers, Supervisors and Veterinary Staff using the AALAS Training Manual series. All staff are members in the local Orange County branch of AALAS and are encouraged to participate in AALAS-sponsored workshops and symposia as well as national and District 8 AALAS meetings. Other Educational Opportunities ULAR schedules educational seminars for husbandry and veterinary staff twice monthly. Seminars are quite varied and range from investigator presentations on unique research models, review of laboratory animal training materials, laboratory animal topics such as microisolator technique, review of ULAR policies, and vendor presentations on new equipment. iii. The Research Team [Guide, pp. 16-17; 115-116; 122; 124] 1) Describe the general mechanisms, by which the institution or IACUC/OB ensures that research personnel have the necessary knowledge and expertise in the animal procedures proposed and the species used. 9 2/11 UCI IACUC policy requires that all individuals who have contact with animals used in research, teaching or related activities be adequately trained in the safe, humane and scientifically acceptable care and use of the animals. This training requirement applies to all investigators, instructors, technical staff, trainees, students, husbandry and visiting personnel who will have any contact with animals. Required training includes the ethical care and use of animals and the regulations, policies and procedures governing animal use in research as well as in the actual procedures that may be performed on animals during the course of the research. UCI uses the web-based training resources of the Collaborative Institutional Training Initiative (CITI) to provide basic training in the humane care and use of animals in research and teaching as well as periodic refresher courses for all research personnel (required of all personnel involved with animal research every 3 years). This basic training is required of all people before they can be approved to work on an IACUC protocol before approval is granted. Handson training is also required to ensure proficiency in all protocolrelated animal procedures and may be obtained from other researchers in the laboratory who are skilled at the procedure in question or by campus veterinary staff. In addition to the basic CITI training modules, researchers must also complete all recommended additional species- or procedure-specific CITI modules and complete an animal facility orientation before gaining access to campus animal facilities. All undergraduate Biological Science students (199 students) must enroll in a mandatory training course that discusses animal care and use, human subject protection and health and safety issues. This course, Biological Sciences 194S, is a prerequisite before 199 students may begin working in laboratories. ORA and ULAR staff are available to provide consultation and assistance to research personnel in areas such as IACUC policies and guidelines, protocol writing/pre-review services, performance and documentation of searches for alternatives to the use of animals, unnecessary duplication and avoidance or minimization of painful or distressful procedures. ULAR staff also provides hands-on training in animal handling and other veterinary procedures to research personnel as needed. a) Briefly describe the content of any required training. The components of required training are: 1) CITI basic course “Working with Live Animals” 2) CITI species specific course(s) 3) CITI pain and distress course. 4) ULAR in-class seminar 5) ULAR animal facility orientation 10 2/11 6) Hands on training conducted by ULAR or other experienced lab personnel b) Describe the timing of training requirements relative to the commencement of work. Completion of all required CITI training modules is required of all personnel before being allowed to work with animals. For specific facility access, personnel must also attend a classroom and animal facility orientation. Hands-on procedural training is normally conducted after new personnel have been added to the protocol and granted access to the facility. c) Describe continuing education opportunities offered. All research personnel must complete CITI refresher training once every three years; compliance is verified at the time of de-novo protocol review. In addition, all existing faculty and staff personnel were required, as part of the transition to photo-identification keycard access to campus vivaria in early 2012, to attend classroom training/facility orientation in order to obtain the new keycards. ULAR and/or the IACUC administrative office offer routine access to workshops, seminars, presentations and webinars offered by Jackson Laboratories, Charles River Labs, OLAW, USDA, etc., on topics ranging from procedural refinements to laboratory security. 2) Describe the process(es) to ensure surgical and related procedures are performed by qualified and trained personnel. Who determines that personnel are qualified and trained for surgical procedures? What role does the Attending Veterinarian and IACUC/OB have in this determination? [Guide, pp. 115-116] The on-line training by CITI has a module in the basic course on anesthesia and surgery and the on-line CITI training has species-specific courses that contain information on anesthesia and surgery for that species. All researchers performing surgery are required to complete these courses as well as the Pain and Distress module from CITI. The required classroom seminar/vivarium orientation also discusses surgery and use of analgesics. The CD produced by NIH Office of Laboratory Animal Welfare is made available to researchers on the ULAR website. ULAR veterinarians are available on an as needed basis to train investigative staff in specific surgical procedures. Investigators that propose surgical procedures in IACUC protocols must document surgical expertise of the personnel 11 2/11 performing the procedures prior to IACUC approval or confirm that appropriate training takes place before the procedures are performed; verification of required skills is a part of the IACUC and ULAR’s veterinary outreach program. This hands-on training may be conducted by ULAR veterinarians or other experienced laboratory personnel. The IACUC may also require that investigative staff attend ULAR sponsored training sessions. To ensure personnel are competent, the IACUC conducts a 6 month inspection of all labs, especially concentrating on labs that perform surgery. ULAR veterinarians perform routine rounds of animals in the facility, frequently examining post surgical animals, and conduct an outreach program that visits labs on an informal, but frequent basis. During these visits surgical procedures are observed and discussed as well as recent outcomes. 3) Describe the training and experience required to perform anesthesia. [Guide, p. 122] See #2 above 4) Describe how the proficiency of personnel conducting euthanasia is ensured (especially physical methods of euthanasia). [Guide, p. 124] The on-line training by CITI has a module in the basic course and in the species-specific courses on euthanasia. All researchers are required to complete these courses. The required in-class seminar and animal facility orientation also discusses euthanasia. ULAR personnel are available on an as needed basis to provide additional training. Finally, investigators must also describe the euthanasia method in their IACUC protocol (including the means by which death is confirmed in euthanized animals), as well as document the expertise of the personnel performing the euthanasia prior to IACUC approval. The IACUC requires scientific justification for physical methods without prior use of anesthetic s for euthanasia. Personnel performing this must be competent and may require that they attend an ULAR sponsored training sessions, if warranted. b. Occupational Health and Safety of Personnel [Guide, pp. 17-23] Describe the institutional entities that are involved in the planning, oversight, and operation of the institutional occupational health and safety program. The responsibility for compliance with applicable regulations concerning hazardous biological, chemical and physical agents rests with UCI Environmental Health & Safety (EH & S) under the direction of Dick Sun, who reports to the Associate Vice Chancellor for Administrative 12 2/11 and Business Services. EH&S, includes departments for biosafety, radiation safety, fire safety, hazardous waste management, injury prevention, industrial hygiene and occupational health. Each School within the University has a dedicated EH & S Coordinator. EH&S also employs a Vivarium/Biosafety Specialist, who is responsible for coordinating all safety matters within the UCI animal facilities; the Vivarium Biosafety Specialist reviews all protocols and modifications submitted to the IACUC for review, and attends all IACUC convened meetings as an ad hoc/non-voting member. The Institutional Biosafety Committee (IBC) is the principal advisory group to the Vice Chancellor of Research on the use of recombinant DNA and biohazardous agents in research. The ULAR Associate Director is a voting member of the IBC. The Laboratory Animal Occupational Health Program (LAOHP) is the joint responsibility of the IACUC and EH&S. Screening information is collected via a web questionnaire located on the ORA-IACUC webpage. Completion of the form is recorded in the IACUC database, but all collected health screening information is forwarded directly to EH&S for review by the Occupational Health Program Coordinator, a registered nurse or Nurse Practitioner. The Occupational Health Program Coordinator reviews all submitted health information and evaluates it with the goal of preventing illnesses related to animal exposure, determining medical clearance or referral and providing a preliminary medical evaluation. In the event that an illness or injury related to work with lab animals is reported via questionnaire, further assessment and treatment is provided through referral to an Occupational Health Care Provider. ULAR includes information about hazards of working with animals in research in the classroom training required for all researchers before facility access is granted. The UCI Workers Compensation Office coordinates medical treatment for workplace injuries. Personnel are directed to the appropriate medical facility, which may be Gottschalk Medical Plaza on the Irvine campus, the Occupational Health Clinic at UCI Medical Center in Orange, Newport Urgent Care (for after hours care), UCI Medical Center Emergency room or the closest Urgent Care Center or hospital Emergency Department. i. Hazard Identification and Risk Assessment [Guide, pp. 18-19; See also Chapters 2 and 3 in Occupational Health and Safety in the Care and Use of Research Animals, NRC 1997] 1) Describe the process used to identify, evaluate and control experimental and other potential hazards (such as ionizing and nonionizing radiation, chemical cleaning agents, animal bites, allergens, zoonoses, and venomous species) inherent or intrinsic to the use of animals by the institution. Describe how risks of these hazards are assessed and how procedures are developed to manage the risks. 13 2/11 Potential hazards are identified by a combination or methods including: 1. Review of IACUC, IBC (Institutional Biosafety Committee), and RUA (Radiation Use Authorization) applications; 2. Laboratory and vivarium visits by EH&S, IACUC and ULAR personnel; 3. Oversight by each school’s EH&S Coordinator, whose duties include identifying and mitigating hazards; 4. Evaluation and mitigation of hazards and risks involved with animal use by the dedicated EH&S Vivarium Biosafety Specialist, who attends monthly IACUC and IBC meetings and coordinates communication between EH&S, IACUC, IBC and ULAR to ensure identification and mitigation of hazards. IACUC protocol review includes review of safety issues, and consultation between the researchers and EH&S to ensure that safe procedures are followed. As a result of IACUC and IBC protocol reviews, Animal Room Hazard Communication sheets are generated for each hazardous agent used in animals. These sheets are posted at the doors of rooms in which hazardous agents are used in animals, so that all personnel have access to the information before they enter the room. Before being added to an animal use protocol, all researchers are required to take the online Collaborative Institutional Training Initiative (CITI) basic animal welfare course, which discusses use of hazardous agents in animals, as well as hazards inherent in working with animals (allergies, bites etc.). Researchers are required to enroll in the Laboratory Animal Occupational Health Program before being added to an animal use protocol. Before being approved for animal facility access, researchers are required to attend a one-hour Researcher Training class taught by ULAR veterinarians. This class includes discussion of hazards inherent in animal use, as well as hazardous agents used in research at UCI. Information includes how to identify enclosures and rooms in which hazardous agents are used. Before a researcher can begin work with hazardous agents, EH&S communicates with the lab to ensure that all personnel are trained in safe use of the hazardous agent. This may include a Safety Considerations meeting with the researchers, animal facility personnel, and EH&S Biosafety Vivarium Specialist. Training of animal facility and research personnel includes use of personal protective equipment, proper safety practices, disposal of animals and animal wastes, decontamination processes, etc. EH&S requires completion of applicable online courses covering “Hazardous Waste”, “Laboratory Core Safety”, 14 2/11 “Bloodborne Pathogens”, “Compressed Gas Safety” and “Respiratory Protection”. Animal husbandry staff are trained by ULAR managers, veterinarians, and EH&S staff in general laboratory and animal facility safety practices, including work with hazardous materials when required. MSDS information for chemical cleaning agents used in the animal facilities is kept in each major animal facility. 2) Describe procedures for reporting and evaluating exposure to hazards, work place injuries, etc. UCI Emergency Procedures Flip-Charts are posted in all work areas, describing procedures for various emergencies, including Hazardous Materials Incidents. For emergencies, personnel are instructed to call 911; on campus, these calls are routed directly to the UCI Police Department Dispatch Center. For non-emergencies, personnel are trained to report incidents to their supervisors after first aid measures are performed. The Supervisor ensures that the UCI Worker’s Compensation Office is notified, and that EH&S is notified via their web portal “Incident Report: Report an Injury, Incident or Safety Concern”. Worker’s Compensation personnel provide EH&S staff with reports of all injuries. EH&S staff then follow-up with personnel for additional safety training, as needed. Evaluating exposure to hazards and workplace injuries related to animal research is the responsibility of UCI EH&S. Use of hazardous agents and other workplace hazards in ULAR facilities are monitored by the Biosafety Vivarium Specialist, who works closely with ULAR managers. The Biosafety Vivarium Specialist meets regularly with ULAR staff, and conducts Safety Considerations meetings with researchers and ULAR staff before hazardous materials are used in animals, so that all personnel with potential exposure are informed about the risks and trained to mitigate them. ii. Facilities, Equipment and Monitoring [Guide, pp. 19-20] 1) Describe how hazardous agents are contained within the study environment and in the animal housing area. Engineering controls such as biological safety cabinets and chemical fume hoods are used as required by EH&S when working with hazardous agents. Rodents exposed to hazardous agents are generally housed in microisolator cages labeled with information about the specific hazard. If ventilated racks are used, negative air flow is established within the cage and the pressure in the room is negative with respect to the 15 2/11 hallway. When animals are infected with biohazardous agents, manipulations are performed either in a biological safety cabinet or with additional PPE as described in the IBC protocol. All animal carcasses are collected as pathological waste and incinerated off-site by a third party vendor. In the ABSL3 facility, all autoclavable materials and rodent carcasses are autoclaved before removal using a pass-through certified autoclave. Bedding and cages may be autoclaved before disposal/washing if they contain infectious agents at Animal Biosafety Level-2 or greater biosafety level, and if deemed necessary by the IBC or Biosafety Officer. Bedding contaminated with hazardous chemicals is bagged and collected by EH&S for disposal. 2) Describe facilities that use hazardous agents. Note square feet/meters, number of animal rooms, and support spaces. In addition, describe design features, construction features, and special equipment, especially as they relate to hazard containment. Note if, and how, exhaust air is treated. If special facilities are not available and animals exposed to hazardous agents are housed within conventional animal rooms, so note. UCI has some specialized facilities for animal studies using hazardous agents: 1. Hewitt Hall includes a fully commissioned and certified Animal Biosafety Level-3 rodent facility (ASF 1,937 including animal housing and use space and support space). The facility has two animal holding rooms and two animal procedure rooms, each with biosafety cabinets. The facility is under negative pressure with an anteroom. The air is single pass and is exhausted to the outside after being HEPA filtered. The facility is also equipped with a restroom and shower, so that the facility may be used to manipulate infectious agents where personnel may be required to shower in and out. The ABSL-3 facility has a certified pass-through autoclave for disinfection of all medical and infectious waste generated within the facility. 2. McGaugh Hall includes a containment suite that is currently used for ABSL2 rodent studies. Total ASF of this suite is 1,097 including animal housing and use space and support space. This suite includes two animal housing rooms, one procedure room, an anteroom, janitorial room and an autoclave room with a pass-thorough autoclave. All autoclavable materials are autoclaved out of this suite. In some cases, animals exposed to hazardous agents are housed within conventional animal rooms. In these cases, enclosures containing hazardous agents are labeled with special cage cards indicating the type of hazard and any special handling required for animals, cages and wastes. 16 2/11 3) Describe the oversight process and husbandry practices in place to ensure personnel safety, including any personal protective equipment provided when work assignment involves hazardous agents. Control of exposure to occupational hazards associated with animal research or handling is achieved through the application of engineering controls, work practices and the use of personal protective equipment (PPE). If engineering controls are not considered sufficient to control the exposure potential, work practices are modified to help minimize exposure potential. PPE (including laboratory coats, disposable gowns, gloves, shoe covers, respiratory protection and eye protection), may be required and is provided at no cost to personnel who need to work with hazardous agents. EH&S conducts periodic inspections to ensure that the required laboratory practices and procedures are followed. Investigators are responsible for ensuring that laboratory personnel are trained in the safe use of hazardous agents. ULAR requires researchers to identify cages in which hazardous agents are used in animals. If an entire housing or procedure room is used for hazardous agent work, the room is posted with applicable warning signs and safety instructions. If only some cages contain hazardous agents, special cage cards are used to identify the hazards and to provide instructions as to how the animals and cages need to be handled safely. Instructions on handling of specific hazardous agents are developed by EH&S, ULAR and the research labs, and communicated to all personnel with potential for exposure. EH&S has developed Standard Operating Procedures regarding safety procedures for the use of some commonly used hazardous agents. 4) Describe any facilities that may also be used for human-based research or patient areas, including the policies and procedures for human patient protection, facility decontamination, animal transport through common corridors or elevators, and other personnel protection procedures. On the Irvine campus, there are two human-size MRI systems and one small animal MRI. Currently, animal and human studies are not performed in the same scanner, although this has been approved by the IACUC. The first human scanner (4T) is solely used for instrumentation development and only animal studies have been performed in the last couple of years. The second scanner, (Philips 3T) has been solely used for human clinical research up to now but recently, animal studies were approved for this scanner, but only if the animal studies 17 2/11 are performed at times when it is not used for humans (at night or during weekends). All of the MR systems are in separate trailers so there are no common elevators or corridors. Procedures are in place to thoroughly disinfect the coil and the patient couch on which the rf-coil holder rests before and after use by animals. At the UCI Medical Center in Orange, the Epidemiology and Infection Prevention (EIP) branch reviews applications for animal use in human clinical settings. IACUC protocols currently approved for animal research in human clinical areas include irradiation (UCI MC irradiation rooms 125 (CT) and 148 (Trilogy) and MRI (UCIMC Building 22). Researchers must submit an application to use animals in clinical areas, and must demonstrate that they have IACUC approval for the work, and that they have procedures planned to prevent crosscontamination of the facilities. UCIMC has a Policy for Animal Research Procedures in Patient Care Areas. This policy includes the following: 1. Instructions on transportation of animals within clinical areas, including a requirement that animals are covered during transportation. 2. Only non-invasive procedures may be performed on animals in clinical areas. 3. Areas used for animal studies must be thoroughly disinfected after use. 4. Animal studies in clinical areas can only be performed during times when clinical use is not needed. 5. One to two hours should elapse between use of areas for animal studies and subsequent clinical use, to allow complete air changes to occur. 6. Any hazardous waste generated during the animal study must be removed immediately after the animal procedure is completed. 5) Describe any other circumstances in which animals or caging equipment are transported in common use corridors or elevators (e.g., have the potential to come in contact with individuals not associated with the animal care and use program), and measures taken to mitigate risks associated with such use. Animals may be transported between the animal facilities and research laboratories for certain procedures. This may involve transportation via common use corridors or elevators. ULAR has a policy that all cages must be covered when being transported outside the animal facilities. ULAR provides disposable, plastic covers which researchers can use to loosely cover cages during transportation. 18 2/11 6) If motorized vehicles are used for animal transport, describe how the driver is protected from exposure to hazards such as allergens or zoonoses. ULAR has three vehicles (a van and two trucks) which may be used to transport animals between facilities on the Irvine campus or between the Irvine and Orange campuses, The van is used for transportation of small animals, such as rodents. The cargo compartment of the van is open to the driver’s compartment. Small animal cages are loosely covered with disposable plastic bags or placed within sanitizable plastic containers during transportation. The larger truck is used to transport large numbers of small animals or any larger animals such as pigs. The cargo compartment of the truck is completely separated from the driver’s compartment. ULAR personnel who transport animals wear scrubs and dedicated shoes. They change scrubs daily, and are encouraged to shower before changing into their street clothes at the end of the work day. Occasionally, small numbers of rodents are transported in personally-owned vehicles (for instance between Irvine and Orange campuses, or to and from other research institutions in Southern California). ULAR has a Standard Operating Procedure outlining procedures for this type of animal transportation, which states that rodents should either be secured into commercially-available transportation containers, or if they are transported in their own cages, the cages must be taped shut and placed within a large plastic bag or sanitizable secondary container. iii. Personnel Training [Guide, p. 20] 1) Describe educational program(s) to inform personnel about zoonoses, personal hygiene, allergies, and other considerations regarding occupational health and safety. - - - The online Collaborative Institutional Training Initiative (CITI) basic animal welfare course and species specific courses, required by all research personnel before they can be added to a protocol, includes modules covering the use of hazardous agents in animals, as well as hazards inherent in working with animals (allergies, bites etc.). The classroom vivarium orientation course required for animal facility access includes specific information about zoonoses, use of hazardous materials in the vivarium, and use of personal protective equipment. The Laboratory Animal Occupational Health Program (LAOHP) website (where the online LAOHP form is located) provides basic information about the hazards associated 19 2/11 - - - - with animal use; additional information and training is offered during personalized follow up provided by Environmental Health & Safety and the Occupational Health Training of animal facility and research personnel includes use of personal protective equipment, proper safety practices, disposal of animals and animal wastes, decontamination processes, etc. EH&S requires completion of applicable online or classroom courses covering “Hazardous Waste”, “Laboratory Core Safety”, “Bloodborne Pathogens”, “Compressed Gas Safety” and “Respiratory Protection”. Animal husbandry staff are trained by ULAR managers, veterinarians, and EH&S staff in general laboratory and animal facility safety practices, including work with hazardous materials when required. Before working in the ABSL3 facility, personnel are required to attend a training course specific to this facility, including hands-on practice in using the PPE required in this facility. 2) Describe special qualifications and training of staff involved with the use of hazardous agents in animals. Personnel working with certain hazards are required to take applicable classes through EH&S (Radiation Safety, Viral Vectors, Bloodborne Pathogens, Compressed Gas Safety, Respiratory Protection Fit Testing. Personnel working in the ABSL3 facility are required to take a class on ABSL3 procedures. iv. Personal Hygiene [Guide, p. 20; Ag Guide pp. 4-5] 1) List routine personal protective equipment and work clothing provided for animal care personnel, technical staff, farm employees, etc. Describe arrangements for laundering work clothing. ULAR provides uniforms (scrub tops and pants) for animal technicians and cage wash technicians to wear in the animal facilities. Animal care and cage wash staff receive an annual stipend to purchase one pair of dedicated work shoes per year. Lockers are provided for storing street clothes. ULAR personnel change into uniforms before working in the animal facilities, and they are encouraged to shower before changing back into their street clothes at the end of the work day. Scrub suits for animal care staff are sent to a commercial laundry each week. Washers and dryers are available in the Med Sci A and McGaugh Hall animal facilities as a back-up if there is a delay in the commercial laundry service. ULAR 20 2/11 personnel are also required to wear the Personnel Protective Equipment applicable to their work areas. Research personnel are required to wear the Personnel Protective Equipment applicable to the animal use area. For most animal facilities, the minimum PPE required to enter is a disposable long-sleeved gown and disposable shoe covers. Certain work areas require additional PPE. Signs are prominently posted to indicate the PPE requirements. 2) Describe provisions for washing hands, showering, and changing clothes, including instances where work clothes may be worn outside the animal facility. Showers and change facilities are located in the major ULAR animal facilities. Sinks are located in some animal holding rooms, all procedure rooms, some corridors and all restrooms for washing hands. Scrub suits may be worn into staff lounge areas, but are not worn outside animal facilities except when staff travels between facilities. 3) Describe policies regarding eating, drinking, and smoking in animal facilities. Smoking is not permitted within any University structure, including animal facilities and laboratories. Eating and drinking within animal facilities is only permitted in designated staff lounge areas. v. Animal Experimentation Involving Hazards [Guide, pp. 20-22] 1) Describe briefly institutional policies governing experimentation with hazardous biological, chemical, and physical agents, including the oversight process for the use of hazardous agents. Note: Written policies and standard operating procedures (SOPs) governing experimentation with hazardous biological, chemical, and physical agents should be available during the AAALAC site visit. If such policies and procedures are not available, please explain. UCI is committed to providing a healthy and safe working environment; University policy delegates responsibility to the Department of Environmental Health and Safety (EH&S) to ensure compliance with all applicable health, safety, and environmental laws, regulations and requirements and to confirm that all activities are conducted in a manner that protects students, faculty, staff, visitors, the public, property and the environment. UCI implements best practices and uses the Integrated Safety and Environmental Management System 21 2/11 (ISEM) to manage health, safety and environmental concerns in all campus activities. - The Institutional Biosafety Committee (IBC) is the principal advisory group to the Vice Chancellor of Research and the Associate Vice Chancellor for Research/Institutional Official on the use of recombinant DNA and biohazardous agents (including infectious agents, human and non-human primate blood, bodily fluids, and tissues) in accordance with the NIH Recombinant DNA Guidelines, Centers for Disease Control and Prevention (CDC) Guidelines, and all Federal, State and local laws. EH&S also includes departments for biosafety, radiation safety, fire safety, hazardous waste management, injury prevention, industrial hygiene and occupational health. - Procedures, guidelines, and other resources to support radiation users on campus help ensure safety and make certain that radiation exposures are reduced to levels that are As Low As Reasonably Achievable (ALARA). Radiation Use Authorization (RUA) must be obtained from the Radiation Safety committee. Radiation Safety training is required for personnel who may be exposed to radiation sources. - All UCI personnel who work with laboratory chemicals must know and follow the standard operating procedures outlined in the UCI Chemical Hygiene Plan. All laboratory operations involving particularly hazardous chemicals must be planned and executed in accordance with the standard operating procedures described in this plan. In addition, each laboratory worker is expected to practice safe personal chemical hygiene habits aimed at reducing exposures to potential hazards. There is a dedicated EH & S Coordinator assigned to each academic school to assist in coordination between laboratories and required safety oversight; EH&S also employs a Vivarium/Biosafety Specialist, who is responsible for coordinating all safety matters within the UCI animal facilities and reviews all IACUC protocols to identify potential hazards. 2) Describe aspects of the health and safety program specifically for personnel potentially exposed to hazardous agents. All animal use protocols submitted to the IACUC and all IBC protocols involving animals are reviewed by the Vivarium/Biosafety Specialist and referred to the appropriate EH&S departments as needed to develop recommendations and training for personnel. If hazardous materials are used as a part of an animal use protocol, Standard Operating Procedures and signs are developed and posted. If procedures are complex or new, a 22 2/11 Safety Considerations Meeting is scheduled in order to review safe handling procedures for animals, equipment and waste materials. EH&S personnel from the appropriate department (e.g. biosafety, radiation safety, industrial hygiene), ULAR personnel and research personnel attend the Safety Considerations Meeting. Topics covered in the meeting may include room entry requirements, review of the safety signage and Standard Operating Procedure, personal protective equipment, Material Safety Data Sheets and exposure control plan, additional training requirements, waste management and medical surveillance. If personnel are immunocompromised due to treatment of certain diseases, or as a result of chronic illness, special considerations may need to be made. The employee or student is encouraged to confidentially discuss the condition with their personal medical provider or the EH&S Occupational Health Program Coordinator. 3) Describe safety procedures for using volatile anesthetics and how waste anesthetic gases are scavenged. If volatile anesthetic agents are administered via a precision vaporizer, waste anesthetic gases are scavenged using an activated charcoal scavenging system, or directed to a building exhaust vent via tubing, or performed within a chemical fume hood or externally-ventilated biosafety cabinet. If volatile anesthetic agents are used without a precision vaporizer, procedures must be performed within in a chemical fume hood, an externally-ventilated biosafety cabinet, or in a well-ventilated area. The method used for scavenging waste anesthesia gases must be described in the approved IACUC protocol and is verified as part of the semi-annual facility inspection visits. 4) List, according to each of the categories noted below, hazardous or potentially hazardous agents currently approved to be used in animals that are or will be maintained for more than a few hours following exposure. If the hazardous agent cannot be listed by name for security/proprietary reasons, identify it by the general category of agent and level of hazard. Note: This information may be provided as an Appendix. a) Biological agents, noting hazard level (CDC Biohazard Level, Directive 93/88 EEC, CDC or USDA/DHHS Select Agent, etc.). Hazards Contained at ABSL1: 1. Adeno-Associated Virus (helper-free) 2. Human cell lines Hazards Contained at ABSL2: 23 2/11 1. Adenovirus 2. Borrelia spp. 3. Chlamydia spp. 4. Herpes Simplex Virus 5. HIV 6. Lentiviral vectors 7. Human Cells 8. Modified Rabies Virus 9. Salmonella spp. 10. Toxoplasma gondii 11. Vaccinia virus Hazards Contained at ABSL3: 1. Prions 2. West Nile Virus 3. Vaccinia Virus b) Chemical agents, noting general category of hazard (toxicant, toxin, irritant, carcinogen, etc.). Toxicants: 1. Azoxymethane 2. Cyanide 3. Kainic acid 4. Streptozotocin Carcinogens: 1. Adriamycin 2. Benzo-a-pyrene 3. Bromodeoxyuridine 4. N-ethyle-N-Nitrosurea 5. Cyclosporine 6. Cyclophosphamide 7. Dimethyl benzanthracene (DMBA) 8. Docetaxel 9. Mitomycin C 10. OH-BBN (N-butyl-butyl-nitrosamine) 11. Paraformaldehyde 12. Rapamycin 13. Tetradecanoyl-phorbol acetate (TPA) 14. Urethane Antineoplastics: 1. Cisplatin 2. Colchicine 3. Cocetaxel 4. Doxorubicin 5. Pacitaxel 6. Streptozotocin 24 2/11 7. Tacrolimus 8. Tamoxifen 9. Temozolomide c) Physical agents (radiation, UV light, magnetic fields, lasers, noise, etc.). 1. 2. 3. 4. 5. 6. Radiation UV light Magnetic fields (MRI) Ultrasound Lasers UV light 5) Describe the program for housing and caring for animals exposed experimentally to the hazardous agents noted above, with emphasis on management and safety practices for containment of each class of agent. Indicate how levels of personnel exposure are assessed. Safety procedures are developed by EH&S, ULAR and the research lab for each hazardous agent used in animals. For biological agents, UCI follows the CDC guidelines as published in the BMBL manual (Biosafety in Microbiological and Biomedical Research, 5th ed.). Typically, all animal work involving ABSL2 or ABSL3 agents is performed within a biological safety cabinet, and rodents are housed in microisolator cages. The minimum PPE required is a disposable gown and disposable latex/nitrile gloves. Additional PPE may be required for some biohazardous studies (including face masks, hair covers, N-95 respirators, surgical face masks). Rabbits infected with Herpes Simplex Virus (HSV) are housed in conventional stainless steel cages. To handle the infected rabbits and tissues, personnel must wear the basic vivarium PPE (disposable gown, shoe covers and gloves, and also a surgical face mask and eye protection (safety glasses or goggles) to protect the mucous membranes. For biohazardous agents, the IBC protocol includes descriptions of how the contaminated wastes and carcasses will be disposed of. For instance, the contaminated cage containing the bedding may be autoclaved before the bedding is disposed of as biohazardous waste. For chemically hazardous agents, the Chemical Hygiene Officer develops an SOP for safe handling of the agents, including instructions for handling animals and their wastes that may be contaminated. Levels of personnel exposure are assessed for certain chemical hazards (e.g. isofluorane) when 25 2/11 required by EH&S, by conducting an exposure assessment for isoflurane for a full shift personal and area monitoring. The lab results are calculated as an 8-hour Time Weighted Average concentration that is compared to the OSHA Permissible Exposure Limits. For animals treated with radioisotopes, the Radiation Safety Officer develops Standard Operating Procedures for safe handling. Procedures are described for handling live animals, their waste materials, carcasses, and soiled cages and other equipment. Depending on the isotope used, procedures may include allowing cages, wastes and carcasses to undergo decay until levels of radioactivity are at a safe level for handling without special precautions. Research staff and animal care staff must undergo radiation safety training if they may be exposed to radioisotopes in the course of their work. Radiation dosimeter badges may be used to determine exposure levels, according to the advice of the Radiation Safety Officer. Hewitt Hall includes a fully commissioned and certified Animal Biosafety Level-3 rodent facility. The facility has two animal holding rooms and two animal procedure rooms, each with biosafety cabinets. The facility is under negative pressure with an anteroom. The air is single pass and is exhausted to the outside after being HEPA filtered. The facility is also equipped with a restroom and shower, so that the facility may be used to manipulate infectious agents where personnel may be required to shower in and out. The ABSL-3 lab has a certified passthrough autoclave for disinfection of all medical and infectious waste generated within the facility. For biohazardous agents, levels of personnel exposure are not routinely assessed, and serum banking of pre-exposure serum is not done. In certain cases, serum titers are measured to determine whether pre-exposure immunization will be recommended or required (e.g. rabies). vi. Personal Protection [Guide, pp. 21-22] 1) Describe training, equipment and procedures employed to reduce potential for physical injury, inherent to animal facilities (e.g., noisy areas, large quantities of chemicals such as disinfectants, ergonomics) or species used (e.g., nonhuman primates, agricultural animals). All UCI employees are advised to view an online New Employee Orientation, a 2-hour tutorial that includes an introduction to safety information and training available to personnel through EH&S. UCI has a program called Safety on Site (SOS) to promote safety awareness in work areas. New employees are required to log on to the University of California Learning Center (UCLC) site and complete a risk assessment 26 2/11 that will determine which training classes they need for their type of work. It is the responsibility of the supervisor / PI to ensure compliance. Animal care staff receives training at staff meetings on ergonomic issues related to their work, including back injuries, repetitive strain injuries and bloodborne pathogen training. All personnel who work with animals must provide evidence of experience and training to safely handle animals and perform their duties, or such training is provided by ULAR. Undergraduate students receive training in laboratory safety by taking Biological Sciences 194S course, which is a requirement for all students entering biological research. This course includes a risk assessment and safety training. Graduate students complete the Teaching Assistants and New Graduate student Orientation (TANGO) course at EH&S. TANGO includes a risk assessment and safety training. Equipment to reduce physical injuries include gloves for handling hot items (from autoclaves or cage washers for instance); carts and pallet jacks for transporting heavy items; aprons and face shields to prevent chemical injury; slipresistant boots in cage wash areas, and hearing protection when required by EH&S. 2) Describe the procedures for the maintenance of protective equipment and how its function is periodically validated. Protective equipment is evaluated regularly by users. Any defects are reported to supervisors, who arrange for protective equipment to be repaired or replaced. 3) Describe situations where respiratory protective equipment is available or required, such as cage washing facilities, feedmills, etc. Describe how such equipment is selected and how respirator fit testing and training in the proper use and maintenance of the respirator is provided. The Respiratory Protection Program is designed to provide for the medical assessment and equipment fit testing as mandated for all employees and students who require the use of a respirator in the scope of their work at UCI. The purpose of the Respiratory Protection Program is to prevent potential health hazards, provide for early treatment of adverse health effects, and to meet regulatory standards as set by the Occupational Safety and Health Administration (OSHA) from exposure to respiratory irritants in the occupational setting. The LAOHP risk assessment is used to identify personnel who may need respiratory protection. Personnel then complete an additional Respiratory Hazard Identification. Based in this evaluation, personnel may be enrolled in the Respirator Fit Test and 27 2/11 Training through EH&S. The appropriate type of respirator is determined and personnel are trained on its correct use. Respirators are provided at no charge to the personnel who require them for their work at UCI. 4) Describe program policies to ensure personnel safety when working with rack/cage washers, other sanitation/sterilization equipment, and other heavy equipment such as scrapers, tractors, and farm machinery. Describe the training program that supports these policies. Training for cage wash personnel is covered by a Standard Operating Procedure. The Cage Wash Training SOP includes directions for training personnel on safety procedures, including: (1) Correct methods to lift, push and pull heavy items (2) Correct use of PPE (3) Handling hot items from autoclaves (4) Locations of emergency exits, eye wash stations, showers, fire extinguishers, and first aid kits (5) Emergency shut-off procedures for equipment such as tunnel washers (6) Emergency shut-off and exit procedures if trapped inside rack washers The UCI animal program does not have large farm machinery. vii. Medical Evaluation and Preventive Medicine for Personnel [Guide, pp. 22-23] 1) Identify the individual(s) and/or office responsible for developing and monitoring the medical evaluation and preventive medicine program. Responsibility for medical evaluation and preventive medicine for personnel (known as the Laboratory Animal Occupational Health Program or LAOHP) is shared between Environmental Health and Safety (EH&S) and the Office of Research. The Occupational Health Program Coordinator or the Employee Health Manager (both within EH&S) review the health risk assessment questionnaires and evaluate the need for medical evaluation by an occupational health physician at the UCI Center for Occupational and Environmental Health (COEH), or respirator fit testing and training within EH&S. Office of Research maintains a database of personnel who are currently enrolled in the LAOHP. At the time of protocol review, the IACUC administrative staff (within Office of Research) check that research personnel are enrolled in the LAOHP. For personnel who are not listed on animal research protocols (e.g. ULAR staff, Facilities Management staff etc.), supervisors within those Departments are responsible for ensuring that their 28 2/11 personnel enroll in the LAOHP if they have significant contact with or exposure to animals. 2) Describe the categories of personnel (research staff, visiting scientists, animal care staff, students, support staff, etc.) included in the program. UCI IACUC policy requires that all faculty, staff, visiting researchers and students who work directly with vertebrate animals or laboratory animal tissues (unfixed animal tissues or body fluids) and Facilities Management personnel who work in animal housing areas participate in the Laboratory Animal Occupational Health Program (LAOHP) through submission of a medical questionnaire. Completion of the questionnaire is required once a year and at the time of a change in health status. Reminders and links to additional information are sent to all research personnel on an annual basis. For other categories of personnel (ULAR and Facilities Management), their supervisors are responsible for ensuring that they complete the LAOHP medical questionnaires. Persons with casual research-animal contact (e.g., IACUC community members and staff that participate in semi-annual Facility Inspections as well as researchers in open labs adjacent to animal use areas, etc.) are also encouraged to participate in the LAOHP by submission of the medical questionnaire. 3) Describe general features of the medical evaluation and preventive medicine programs, including pre-employment/pre-assignment health evaluation, periodic medical evaluations, immunization programs, and procedures for communicating health related issues. Screening information is collected via a web questionnaire located on the ORA-IACUC webpage (see Appendix 5. Completion of the form is recorded in the IACUC database, but all collected health screening information is forwarded directly to EH&S for review by the Occupational Health Program Coordinator. The Occupational Health Program Coordinator reviews all submitted health information and evaluates it with the goal of preventing illnesses related to animal exposure, determining medical clearance or referral and providing a preliminary medical evaluation. In the event that a health risk related to work with laboratory animals is reported via questionnaire, further assessment and treatment is provided through referral to an Occupational Health Care Provider at the UCI Center for Occupational and Environmental Health (COEH). Tetanus immunizations are recommended for all animal handling staff, in accordance with the guidelines of the 29 2/11 Federal Centers for Disease Control. For projects involving the use of certain ABSL2 agents (rabies, vaccinia), serum antibody titers and immunization may be recommended for personnel with the potential for exposure to these agents. Health risks of working with animals are communicated in multiple ways. Research personnel have to take online and classroom training before being granted access to ULAR animal facilities. This training includes discussion of health issues related to animal studies. Animal care personnel receive classroom training on health issues, coordinated by the UCI Biosafety Vivarium Specialist. Posters within the animal facilities notify personnel of certain health issues such as animal bites and sharps injuries. For research projects involving hazardous agents, EH&S arranges Safety Considerations meetings with personnel potentially exposed to the hazards before the work can be started. Animal Room Hazard Communication sheets are posted at the doors or animal rooms in which hazardous agents may be used. 4) Describe special precautions or procedures for personnel exposed to potentially hazardous species (nonhuman primates, sheep, etc.) or agents (infectious agents, human origin tissues, chemicals/toxins, etc.). No research involving non-human primates or sheep is currently performed at UCI. c. Investigating and Reporting Animal Welfare Concerns [Guide, pp. 23-24] Describe institutional methods for reporting and investigating animal welfare concerns. Any individual may report concerns to the IO, IACUC Chair, Attending Veterinarian or any member of the IACUC. In addition, complaints or concerns regarding the care and use of animals are directed to the IACUC administrative staff or to other members of the Office of Research where they are documented and kept confidential. There is also a method in place for individuals to leave an anonymous telephone message. Concerns may be reported either verbally or in writing. Notices are located in all animal housing and use areas advising individuals how and where to report animal concerns; such information is also provided on ULAR and Office of Research websites and reiterated in all animal-related training. University whistleblower policy protects any individual against reprisals who, in good faith, reports an animal concern. Upon receipt of a concern or complaint, IACUC administrative staff may perform an administrative audit with assistance of campus veterinary staff as well as other members of the Office of Research Administration to determine whether a possible occurrence of non-compliance 30 2/11 warrants further attention. Substantiated occurrences of noncompliance will be immediately reviewed by the IACUC Chair and Attending Veterinarian, who determine whether immediate action is required (e.g., temporarily halting animal use activity pending formal review by the IACUC.) All reported concerns, regardless of the nature, are brought to the attention of the full committee. However, unless immediate IACUC action is warranted, the Committee is generally informed at the next regularly scheduled meeting. Compliance issues are a standing item on the monthly agenda. B. Program Oversight 1. The Role of the IACUC/OB [Guide, pp. 24-40] a. IACUC/OB Composition and Function [Guide, pp. 17; 24-25] Please provide a Committee roster, indicating names, degrees, membership role, and affiliation (e.g., Department/Division) as an appendix. i. Describe Committee membership appointment procedures. Nominees for membership to the IACUC are submitted to the Associate Vice Chancellor for Research/Institutional Official, who reviews the qualifications of the nominees and makes the appointments to the IACUC. The IACUC Chair is also appointed by the Associate Vice Chancellor for Research. Membership needs of the committee are regularly reviewed by IACUC administrative staff and communicated to the IO to ensure that the committee remains appropriately constituted. ii. Describe frequency of Committee meetings. Regular convened meetings with a quorum of members take place monthly, on the second Thursday of the month. Two additional meetings, on the fourth Thursday in January and July, are reserved for the semi-annual Program Evaluation. iii. Describe the orientation, training, and continuing education opportunities for IACUC/OB members. [Guide, p. 17] New members appointed to the IACUC are provided with copies or web-based links to all relevant laws and regulations, institutional policies and procedures, and The Guide for the Care and Use of Laboratory Animals, and are asked to complete the “Essentials for IACUC Members” module provided by the Collaborative Institutional Training Initiative (CITI) prior to attendance at their first IACUC meeting. New members are mentored by more seasoned committee members and given an opportunity to observe the workings of the committee, including protocol review, for at least 31 2/11 two months before being assigned primary protocol review responsibilities. Additional training for new and continuing members is provided by Office of Research Administration staff during regularly scheduled committee meetings and via a “member’s only” website. Continuing education in the form of articles, newsletters, etc., is routinely provided as an agenda item at convened meetings for review and discussion. In addition, all committee members are encouraged to take part in outside training such as PRIM&R conferences and IACUC 101. b. Protocol Review [Guide, pp. 25-26] A blank copy of your institution’s protocol review form should be provided as an appendix. Also include forms used for annual renewal, modifications, amendments, etc., as applicable. i. Describe the process for reviewing and approving animal study protocols, including research and teaching proposals. Include a description of how animal study protocols that do not involve a formal grant proposal are reviewed and approved (i.e., pilot studies or internally funded studies). Include a description of how the IACUC/OB weighs the potential adverse effects of the study against the potential benefits that may result from the research. Describe how protocols that have the potential to cause pain or distress to animals are reviewed, alternative methodologies reviewed, veterinary input solicited, and studies controlled or overseen. Specify how animals and experimental group sizes are justified. Prior to beginning proposed activities involving the use of live vertebrate animals, and upon the three-year anniversary of approval of ongoing research projects, researchers must complete a protocol application and submit it to the IACUC office. All new and three-year renewal applications (with two exceptions noted below) require full committee review at a convened meeting with a quorum present. Once the submission deadline has passed, all submitted materials are routed to campus veterinarians and EH&S staff for pre-review. Administrative, veterinary and hazard-related review comments are then compiled and posted with the protocols and modifications submitted for full committee review on a protected website and an announcement is made to all IACUC members that materials are ready for review. A primary and secondary reviewer is assigned for each protocol on the agenda. IACUC reviewers submit their review comments back to the IACUC administrative staff, who then compiles them with the veterinary and other pre-review comments for forwarding to the Lead Researcher. Lead Researchers then have approximately 5 days to respond to the pre-review comments prior to final review at the convened meeting. During the Committee meeting, the primary and secondary reviewers are responsible for leading the discussion with regard to the proposed research, summarizing the issues cited in pre-review and assessing the researchers’ responses to those 32 2/11 issues as well as making a recommendation for approval, require modifications in (to secure approval), or withhold approval. If modifications are required, the quorum of members present at the full committee meeting determines if those modifications can be reviewed by Designated Member Review (DMR) or must be returned for review by the full committee at a convened meeting. All members of the IACUC have agreed, as part of the Member Standards document signed at the time they join the IACUC that the quorum of members present at a convened meeting may decide by unanimous vote to use DMR subsequent to full committee review when modifications are required to secure approval. Exceptions to full committee review for new and three-year renewal applications: (A) Research involving animals that are studied in their natural habitat without any invasive procedures and without harming or materially altering their behavior (non-invasive field studies) may qualify for Designated Review; and (B) Research performed entirely offsite at an OLAW-assured institution. UCI’s IACUC may accept registration of the offsite institution’s IACUC approval in lieu of review and approval by the UCI IACUC, following submission of the off-site entity’s approved protocol and approval letter for review by the IACUC chair or designee. All animal study protocols are reviewed following the same procedures and considerations, regardless of whether they are supported by extramural funds. In cases where there is no extramural funding, IACUC members may apply additional rigor to the review to evaluate scientific elements of the protocol as they relate to the welfare and use of the animals. The appropriateness of the chosen species and the experimental group sizes must be carefully justified; researchers are encouraged to provide statistical support for the proposed experimental design wherever possible. The IACUC routinely assesses the potential benefits of every study in light of the potential harm to its animal subjects. In order to gain approval, animal use protocols must clearly document consideration of alternatives, refinement of procedures, and appropriate use of mitigating factors such as anesthesia, postprocedural analgesics, and humane endpoints. Veterinary staff members review all protocols and regularly consult with researchers to ensure animal welfare; the IACUC may also require enhanced veterinary oversight as a condition of approval for protocols that have the potential to cause pain, distress or discomfort in the animals. ii. Describe process for reviewing and approving amendments, modifications, and revised protocols. If applicable, include a description of “major” vs. “minor” amendments. 33 2/11 Review and approval of significant changes to approved protocols require full committee review (FCR) at a convened meeting and are handled in the same manner as new and de-novo renewal protocol applications (see B.1.b.i above). The IACUC has established criteria for the types of significant protocol modifications suitable for Designated Member Review (DMR). These include minor increases in the number of animals allocated to the project (i.e., 10% or less of the originally approved allocation); refinements in the anesthesia regimen; addition of an AVMA approved euthanasia method; decrease in the invasiveness of a procedure; modification to decrease pain or distress; addition of a minimally-invasive procedure or any other changes deemed appropriate by the full IACUC. Modification requests are reviewed administratively; if they are determined to be suitable for DMR, the modification request and all associated documents are forwarded to all members of the IACUC electronically for review. Committee members have five (5) business days to review the request, ask questions and request additional information if needed; during this review period, if any member calls for full committee review (FCR) of the request, it is placed on the agenda for the next scheduled IACUC meeting and the Principal Investigator is notified. If no one calls for FCR during the review period, concurrence by silent assent of all members is assumed and a subcommittee consisting of at least one scientific member and one veterinarian is assigned to review the modification request. The subcommittee may approve, require modifications (to secure approval) or call for full committee review of the modification request. If multiple designated reviewers are used, their decisions must be unanimous; if not, the protocol will be referred for FCR. Minor modifications (e.g., addition or deletion of non-key personnel such as graduate students, change in laboratory location) are reviewed and processed administratively. A list of modifications approved by subcommittee via DMR or administratively review is provided to IACUC members for review and appended to the minutes of the next convened IACUC meeting. c. Special Considerations for IACUC/OB Review [Guide, pp. 5; 27-33] i. Experimental and Humane Endpoints [Guide, pp. 27-28] Describe how criteria for determining alternatives to experimental (humane) endpoints are developed, approved, and applied. Include a description of monitoring systems in place for studies for which information on alternative endpoints are not available. The IACUC requires that every animal-use protocol include welldefined, humane endpoints for all experimental studies to ensure the minimization of animal pain and distress. Criteria have been established and are publicly available via the internet to assess 34 2/11 species-specific signs of pain, distress and discomfort such as weight loss, weakness, anorexia/cachexia, etc. In addition, specific guidelines are provided for tumor studies to ensure that tumor burden does not exceed acceptable levels. Wherever feasible, investigators are asked to develop a scoring system to assess pain and distress in the animals. When questions regarding the appropriateness of established endpoints are noted during protocol review, the IACUC requests that the protocol be flagged for enhanced veterinary oversight and outreach in order to ensure that humane experimental endpoints are established and followed. ii. Unexpected Outcomes that Affect Animal Well-being [Guide, pp. 2829] Describe how unexpected outcomes of experimental procedures (e.g., unanticipated phenotypes in Genetically Modified Animals) are identified, interpreted, and reported to the IACUC/OB. IACUC guidelines have been developed to encourage open communication between research personnel, ULAR veterinarians and the IACUC. Researchers are encouraged to promptly report adverse events, including unanticipated morbidity, injury or pain/distress as well as unusually high frequency of expected clinical signs associated with study procedures. Campus veterinary staff works with researchers to troubleshoot experimental procedures and develop alternative methods to address issues as they arise. These issues are reported to and discussed with the IACUC at the monthly convened meeting as part of the Veterinarian’s Report, a standing agenda item. iii. Physical Restraint [Guide, pp. 29-30] Note: This section is to include only those protocols that require prolonged restraint. Brief restraint for the purpose of performing routine clinical or experimental procedures need not be described. 1) Briefly describe the policies for the use of physical restraint procedures or devices. IACUC policy requires that the use of prolonged restraint be scientifically justified; that the period of restraint should be the minimum required to accomplish the research objectives; that animals should be adapted to the restraint device wherever feasible prior to the actual experiment; and that animals under restraint be monitored and observed for distress at appropriate intervals (as described in detail in the approved protocol). 2) Describe animal restraint devices that are used or have been used within the last three years. For each device, briefly describe the 35 2/11 duration of confinement, acclimation procedures, monitoring procedures, criteria for removing animals that do not adapt or acclimate, and provision of veterinary care for animals with adverse clinical consequences. Most restraint currently used at UCI is minor in nature: - Alligators, snakes and other reptiles are held in metabolic chambers large enough for the animals to fit in a natural posture for periods of up to four weeks. These species are relatively sedentary in nature; it is believed that such restraint is not particularly stressful for them - Aquatic turtles are used in a forced-submersion experiment where they are held underwater for an indeterminate time (they are released 60 seconds after they begin to forcefully try to return to the surface of the water. - Rodents are held in various restraint devices (conical tubes to elicit a stress response, brief restraint for various procedures, etc). A few currently approved studies include restraint procedures that may involve more potential for distress: - Goldfish are held in a custom-made Plexiglass holder for electrophysiology recording and brain imaging. General anesthesia is not used as it would disturb the pattern of brain activity under study, but local anesthesia is given prior to the surgical preparation for the imaging procedures. The animals are kept moist and “ventilated” via a continuous flow of water over the gills. Animals are held in this manner for up to 6 hours and then euthanized. - There is currently one approved protocol that uses the hindlimb suspension model in rats as a simulation of zerogravity conditions. Animals are suspended by the tail from the top of the cage so that only their front limbs bear weight for up to 7 days; cage bottoms are specially designed (sloped) to assist in maintaining animals’ posture as horizontal as possible without enabling the hind limbs to contact surfaces with weight-bearing force. Animals are not acclimated prior to the procedure, but are carefully monitored by both laboratory staff and animal-care personnel. Animals that develop a necrotic tail or show overt signs of pain or distress are euthanized immediately. iv. Multiple Survival Surgical Procedures [Guide, p. 30] Note: One survival surgical procedure followed by a non-survival procedure is not included in this category. 1) Describe the institutional policy(ies) regarding multiple survival surgery (major or minor) on a single animal. 36 2/11 IACUC policy discourages the use of multiple major survival surgery, but it may be permitted if there is a strong scientific justification. The multiple surgeries should be interrelated components of the same project. 2) Describe the procedure for approving multiple survival surgery (major or minor) and the criteria used to determine the potential impact on the animals’ well-being. Protocols which included multiple surgeries are carefully considered by the IACUC, with particular attention to the timeframe of the multiple procedures, the post-operative care provided to the animals and appropriate recordkeeping throughout the duration of the study. The IACUC may request progress reports from principal investigators and/or veterinary staff to assess the health and well-being of the animals. 3) Summarize the protocols currently approved that involve multiple major survival surgical procedures and the time allowed between procedures on the same animal. Describe the method of institutional monitoring. Prot. # 1999-2064 Summary of Surgical Procedures Burn injury followed by biopsies in rodent 1999-2128 2001-2259 2002-2365 2003-2498 2007-2705 2007-2754 2008-2846 Oocyte harvest (total 6) in frog Sciatic nerve transection – 2 procedures (rodent) Spinal cord injury followed by surgical injection (rodent) Sciatic nerve injury followed by surgical treatment Multiple limb amputation in axolotl Multiple tracheal scrape injury in rabbits Implantation of temperature logger followed by implantation of blood flow probe and vascular catheter in alligator Aortic occlusion, followed by implantation of flow probes in alligator Tumor implantation followed by laser treatment in rat Spinal cord injury followed by surgical injection in rodent Brain injury followed by cranial injection in rodent Tumor implantation followed by tumor resection in rat Laminectomy followed by repair surgery in rat Intracranial injection, headplate implant, eyelid closure in mouse Microinjections into subarachnoid space in rat Islet transplant into kidney or liver, followed by removal of implant in rodent 2009-2876 2009-2895 2010-2940 2010-2945 2010-2959 2011-2976 2011-2994 2011-3005 2012-3028 Interval between surgeries 1, 2,7 and 14 days after injury At least one month 1 week 4 weeks – 1 year 3-6 months 48 hours to 2 weeks 2 days and 4 days 3 months 2-7 years 10 days 9 days 1-2 weeks 10 days 4 weeks 1-45 days 5-8 days 4 weeks Depending on the severity/invasiveness of the procedure, the IACUC may require enhanced veterinary oversight of animals that undergo multiple survival surgical procedures. In all cases, the justification for the multiple procedures is carefully evaluated by the IACUC. v. Food and Fluid Regulation [Guide, pp. 30-31] 1) Describe experimental situations that require food and/or fluid regulation. Note: This does not include pre-surgical fast. List title of 37 2/11 the experiment(s), justification, species involved, and length and type of food/fluid regulation. Prot. # 1997-1608 Species Rodent 1997-1965 Rat 1998-1301 1998-1306 Rodent Rat 1998-1334 Finch 1998-2015 Rat 1999-1719 Rodent 1999-2086 Rat 2000-2165 Rat 2000-2220 2001-2259 2002-2365 2004-2514 2004-2554 2006-2620 Rodent Rodent Rodent Rat Fish Mouse 2006-2630 Rat 2006-2636 Rat 2006-2649 2006-2666 Mouse Mouse 2009-2866 Rat 2009-2867 Rat 2009-2879 Rat 2010-2919 Rat 2010-2945 Rodent 2011-2980 Rodent 2011-2994 Mouse 2011-3009 Rat Reason and Duration of Restriction Water restriction to train/motivate animals for behavior tests. 4 days, 10 ml water/day Food restriction to stimulate exploratory behavior during self-administration of test agents. Maintain animals at 90% of normal body weight. Overnight fasting to prevent contamination of urine sample collection. Water restriction for motivation in reward training. Access to water limited to 10 min / day during training period Withhold food overnight to motivate animals for behavior tests (4:00 p.m. – 9:00 a.m. daily) Food restriction to increase motivation for behavior testing. Food reduced by 20%. Weight loss not to exceed 15% Food restriction to increase motivation for behavior testing. Maintain animals at 80% of normal (age matched) body weight Food restriction to increase motivation for behavior testing. Maintain animals at 85% of normal (age matched) body weight. Water restriction for Vogel testing (water removed at 5:00 p.m. night before testing). Day of testing only. Food restriction to increase motivation for behavior testing, 18-24 hours. Food restriction to increase motivation for behavior testing (overnight). Food restriction to increase motivation for behavior testing (overnight). To determine effects of feeding on OEA biosysthesis in brain (up to 24 hours). Adult zebrafish are fasted to enhance predator-prey experiments (6 days). Food restriction to increase motivation for behavior testing. Maintain animals at 85% of normal (age matched) body weight. Food restriction to increase motivation for behavior testing. Animals are fed after testing and allowed to maintain normal weight. Food restriction to increase motivation for behavior testing. Maintain animals at 85% of normal (age matched) body weight. 24 hour fast for increased glucogenesis. Food restriction to increase motivation for behavior testing. Maintain animals at 85% of normal (age matched) body weight. Animals are food-deprived prior to the sham-feeding procedure described in the approved protocol (18-24 hours). Food and water restriction to increase motivation for behavior testing. Maintain animals at 85% of normal (age matched) body weight. Food restriction to increase motivation for behavior testing. Maintain animals at 85% of normal (age matched) body weight. Food restriction to increase motivation for behavior testing. Access to food limited to daylight hours and one hour during dark cycle. Water restriction to train/motivate animals for behavior tests. Access to water limited to 15 minutes twice daily for 5 days. Food is withheld for 24 hours prior to euthanasia/tissue harvest in study of MCH system. Water restriction to train/motivate animals for behavior tests. Access to water limited to 2 hours/day Food and water restriction prior to sucrose preference test (to assess depressive behavior) – 20 hours. 2) Describe animal health monitoring procedures and frequency (e.g., body weight, blood urea nitrogen, urine/fecal output, food/fluid consumed). The primary variable measured to assess animal health during food or fluid restriction in rodents is body weight. In general, weight loss is limited to no more than 20% of age-matched 38 2/11 controls. Researchers are required to include a detailed description of their monitoring procedures, including the frequency with which animals are weighed, in their approved protocol. Husbandry and veterinary staff observe animals daily for signs of distress such as ruffled coat, hunched posture, obvious/significant weight loss, lack of urine/fecal output and visual signs of dehydration. 3) Describe methods of ensuring adequate nutrition and hydration during the regulated period. Researchers are required to maintain a daily log sheet in the animal room at all times when food or water is withheld. The log must include a record of when food or water is provided, notes about the health status of the animals (including weight) and any adverse events associated with the restriction. The log must be completed by laboratory staff on a daily basis, including weekends and holidays. Husbandry and/or veterinary staff check the logs daily; if the log is incomplete, staff will provide the animals with food and water. A sample log is available for researcher use on the IACUC website. vi. Use of Non-Pharmaceutical-Grade Drugs and Other Substances [Guide, p. 31] Describe the rationale and consideration given by the IACUC/OB for use of non-pharmaceutical grade drugs or other substances, if applicable. IACUC policy requires the use of pharmaceutical grade drugs whenever they are available, in all situations where the health and well-being of the animal are at risk (e.g. anesthesia, analgesia, emergency drugs and euthanasia agents) and for routine veterinary care. Under certain circumstances, however, the IACUC may approve the use of non-pharmaceutical grade chemical compounds with adequate scientific justification or when an acceptable veterinary or human pharmaceutical-grade product is unavailable. In such cases, the method of preparation of the drug and the storage conditions must be described in the animal-use protocol; in particular, a detailed description of the methods used to ensure sterility of the drug. vii. Field Investigations [Guide, p. 32] Describe special considerations used by the IACUC/OB when reviewing field investigations of animals (non-domesticated vertebrate species), if applicable. Field studies are uncommon at UCI; when they are proposed, the IACUC reviews the protocol in the same manner as any other protocol. Such protocols are also carefully reviewed by 39 2/11 Environmental Health and Safety to identify hazards associated with the research or the location. IACUC and ULAR staff also works with the research staff to ensure that all required permits (e.g., California Department of Fish and Game) are in place prior to commencement of the study. viii. Agricultural Animals [Guide, pp. 32-33] Describe considerations given and guiding documents used by the IACUC/OB when reviewing “biomedical” and “agricultural” research projects involving agricultural species as study animals, if applicable. UCI does not perform any agricultural research with animals. ix. Animal Reuse [Guide, p. 5] Describe institutional policies and/or oversight of animal reuse (i.e., on multiple teaching or research protocols). Summarize the protocols currently approved that involve the reuse of individual animals. Individual animals are not reused under multiple teaching or research protocols, with the possible exception of the ULAR protocol “Education and Training in the Use of Laboratory Animals” which will occasionally re-use the same animal to demonstrate minimally-invasive techniques such as blood draw and suturing to multiple groups. On occasion, the IACUC will approve a secondary use of an animal either immediately before or after euthanasia on a terminal procedure – for example, harvest of an organ from swine used in terminal Urology teaching protocols. This type of request is reviewed and approved by the IACUC on a case-by-case basis. 2. Post-Approval Monitoring [Guide, pp. 33-34] a. Describe mechanisms for IACUC/OB review of ongoing studies and periodic reviews (e.g., annual review, 3-year renewals if PHS funded, etc.). All protocols are reviewed by a member or members of the IACUC at least annually. Protocols are approved by the IACUC for a period of up to 365 days. Prior to the expiration date of the project, investigators are notified that a short application for annual continuation must be reviewed and approved by the IACUC before the protocol expires. Protocols are considered for annual continuing review twice; i.e., at the end of the first and second protocol year, before they must be resubmitted for full committee review. All IACUC members receive a list of all expiring protocols one month prior to the expiration date. Any member of the IACUC may obtain, upon request, full committee review of a project scheduled for annual continuing review. If full committee review is not requested and no significant changes are noted on the annual continuation application, a subcommittee of one (e.g., the IACUC Chair or designee) reviews the project and has the delegated 40 2/11 authority to approve, require modifications or refer the submission for full committee review. A list of protocols scheduled for annual review in the upcoming month is included in each month’s meeting agenda and appended to the IACUC meeting minutes. Any IACUC member can call for Full Committee Review of any protocol scheduled for annual/continuing review. If an investigator wishes to continue animal use beyond the end of the third year, a new protocol application must be submitted, reviewed and approved as described above (Section 2.I.B.1.b.i) prior to expiration of the original or preceding protocol (de-novo review). If the de-novo protocol is not reviewed and approved prior to the preceding protocol expiration date, all animal use must cease and may not resume until a new protocol approval is in place. De novo review is required of all protocols after three years, regardless of the source of funding. b. Describe the process and frequency with which the Committee reviews the animal care and use program and conducts facility and laboratory inspections. Detail any criteria used for exempting or varying the frequency of reviewing satellite holding facilities and animal use areas. If contract facilities or contractor-provided personnel are used, describe procedures used by the IACUC/OB to review such programs and facilities. Note: A copy of the last report of these reviews should be included as an appendix. The IACUC meets twice per year outside of regularly-convened meetings to review the Institutional Program for Humane Care and Use of Animals, using the Guide for the Care and Use of Laboratory Animals, PHS Policy and Code of Federal Regulations as a basis for the review. A checklist based on the Sample OLAW Program and Facility Review Checklist is also used to facilitate the evaluation. The evaluation typically includes a review of IACUC membership and functions, records and reporting requirements, husbandry and veterinary care, personnel qualifications and training, occupational health and safety and disaster planning. Facility inspections are performed in May and November of each year. Subcommittees of the IACUC consisting of at least two voting members visit all facilities where vertebrate animals are housed or used; i.e. holding areas, animal care support areas, storage areas, procedure areas and laboratories where animal manipulations are conducted. (Note: Laboratories where animals are used in non-surgical/noninvasive procedures but not housed (as delineated in 9CFR and the PHS Policy) may be visited once per year. The site-visit subcommittees take full advantage of the opportunity to interface with research personnel, review approved procedures, make recommendations and hear investigator concerns. If deficiencies are noted either during the Program Review or Facility Inspections, they will be categorized as significant or minor and the Committee will develop a reasonable and specific plan and schedule for correction. 41 2/11 c. Describe institutional responses to deficiencies noted on regulatory inspection reports (e.g., government, regulatory agencies). Note: Copies of all such inspection reports for the past three years (if available) should be available for review by the site visitors. USDA-APHIS Inspection Report dated June 7, 2011 noted significant cracking and chipping of floor surfaces in the cage-wash area of one vivarium facility. A correction date of September 7, 2011 was assigned for correction of the deficiency. The floor was completely replaced prior to the correction date. d. Describe other monitoring mechanisms or procedures used to facilitate ongoing protocol assessment and regulatory compliance. The IACUC’s monitoring and outreach program involves a variety of oversight activities to assure animal welfare and compliance with approved protocols, including: - Centralized animal ordering and tracking of animal numbers - Regular veterinary rounds to observe animals, particularly following invasive procedures. - Periodic veterinary review of medical records - Regularly-scheduled facility inspections - Periodic re-inspection of approved activities performed in follow-up to routine facility inspections - Enhanced veterinary oversight as requested by the IACUC at the time of initial protocol review. - For-cause protocol review by the IACUC in response to animal welfare concerns or reports of possible non-compliance. II. Animal Environment, Housing and Management Note: Complete each section including where applicable, procedures performed in farm settings, field studies and aquatic environments, etc. A. Animal Environment 1. Temperature and Humidity [Guide, pp. 43-45] a. Describe briefly the heating and air conditioning system performance. Provide method and frequency for assessing, monitoring, and documenting animal room or housing area temperature and humidity that is appropriate for each species. Note current (measured within the last 12 months), detailed (by room) performance data are to be provided as indicated on the enclosed Heating, Ventilation, and Air Conditioning (HVAC) System Summary appendix. If outdoor housing areas are used, so note. Room high and low temperature and current humidity are observed and recorded daily for each animal housing room. Observations are 42 2/11 recorded on individual room maintenance sheets by the husbandry staff. Current room ventilation data is provided in Appendix #10. This is assessed once every three years by ULAR husbandry or facility HVAC personnel using a balometer and assessing air pressure differential at the room entry door. Within the past 3 years most of the animal facilities had an HVAC air balancing update performed. In addition, many of the animal facilities are continually monitored centrally by physical plant personnel. If temperatures vary outside ranges defined in the Guide, alarms are activated in central plant to alert staff 24 hours per day. b. If temperature set points and/or environmental conditions are outside the thermoneutral zone for the species, describe the process for ensuring behavioral thermoregulation (e.g., nesting material, shelter, etc.) and/or IACUC/OB approved exception. Rats for one researcher are held at 6ºC for 8 weeks. This is part of an approved IACUC protocol to induce cold stress. Rats are pair housed and provided with “crinkled paper” for nesting. Rats’ health is monitored daily, weight 3 times per week, and blood pressure once weekly. 2. Ventilation and Air Quality [Guide, pp. 45-47] a. Briefly describe the performance aspects of the ventilation system. Provide method and frequency for assessing, monitoring, and documenting the animal room ventilation rates and pressure gradients (with adjacent areas). Note: current (measured within the last 12 months) detailed (by room) information is to be provided as indicated on the enclosed Heating, Ventilation, and Air Conditioning (HVAC) System Summary appendix. Ventilation in the animal rooms is 100% non-recirculated (with the exception of 4 rooms noted in c below), course-filtered fresh air, maintained within 2ºF of the temperature set point. Animal housing and cage wash rooms are ventilated to maintain a minimum of 10 ACH with procedure rooms at a minimum of 6 ACH. Differential air pressure is balanced for each room as positive to exclude (i.e.: clean cage wash and many transgenic mouse holding rooms) or as negative to retain possible pathogen spread (i.e.: necropsy rooms, biohazard rooms, soiled cage wash). Upon request, UCI Facilities Management adjusts the animal room ventilation rates and/or pressure gradients. b. Describe ventilation aspects of any special primary enclosures using forced ventilation. The majority (95% +) of mice and a small percent of rats are housed in individually ventilated cages. The caging systems include HEPA-filtered supply air to each cage and either HEPA-filtered exhaust air (which in most rooms is vented into the building exhaust) or is directly vented into the building exhaust without being HEPA filtered. Most of the cages are 43 2/11 operated as positive pressure, with cages in ABSL2 areas operated as negative. c. If any supply air used in a room or primary enclosure is recycled, describe the percent and source of the air and how gaseous and particulate contaminants are removed. 4 rooms in McGaugh Hall house reptiles that require a warmer temperature. In order to maintain the warmer temperature, these rooms utilize a recirculating system that maintains 10 ACH, but 65-75% of this air is recirculated back into the room of origin. 3. Life Support Systems for Aquatic Species [Guide, pp. 84-87] Provide a general description of institutional requirements for enclosures using water as the primary environmental medium for a species (e.g., aquatics). Describe overall system design, housing densities, and water treatment, maintenance, and quality assurance that are used to ensure species appropriateness. Please note that facility-specific tank design and parameter monitoring frequencies should be summarized in the Aquatic Systems Summary appendix. Various aquatic housing systems are used at UCI, including simple static tanks and re-circulating aquatic housing systems. For ULAR-maintained aquatic species (currently limited to amphibians), ULAR has Standard Operating Procedures for husbandry of the different amphibian species. These SOPs include water quality parameters, water treatment specifications, quality assurance procedures, stocking densities etc. Laboratories that provide husbandry for their aquatic animals are also required to have written SOPs describing the husbandry, water quality parameters, quality assurance procedures, stocking densities etc. UCI IACUC has a policy on the Care and Use of Ectothermic Vertebrates, which includes some general guidance for housing aquatic vertebrates. 4. Noise and Vibration [Guide, pp. 49-50] Describe facility design features and other methods used to control, reduce, or prevent excessive noise and vibration in the animal facility. Service areas are located away from the animal rooms. Noisy species such as cats and pigs are housed as far away as possible from rodents. If repairs or construction is required within an animal facility, attempts are made to relocate animals away from the noise and activity associated with such work. B. Animal Housing (All terrestrial, flighted, and aquatic species) 1. Primary Enclosures 44 2/11 Provide a description of primary enclosures used (e.g., cages (conventional, individually-ventilated cage systems (IVCS), etc.), pens, stalls, pastures, aviaries, tanks) in appendix. a. Describe considerations, performance criteria and guiding documents (e.g. Guide, Ag Guide, ETS 123 and/or other applicable standards) used by the IACUC/OB to verify adequacy of space provided for all research animals, including traditional laboratory animal species, agricultural animals, aquatic species, and wildlife when reviewing biomedical, field and agricultural research studies. For all animal studies the Guide is used to ensure all animals are provided with adequate space. b. Describe space exceptions to the guiding documents (Guide, Ag Guide, ETS 123, and/or applicable standards), indicating the references, considerations and performance criteria used (e.g., by the IACUC/OB) to verify adequacy of space provided for all animal species covered by the program. [Guide, pp. 5563] Performance criteria are used in determining space requirement in certain instances. For mice, we have determined that it is acceptable to have more than one litter at a time in a cage as long there are no more than 14 pups total and all pups are within 5 days of age. For rats with litters, our cages are 144-147 in2. We typically leave the male in with the nursing female so the male does not need to be single housed. Our rat breeding is typically mutant or transgenic rats, and litter size typically is in the 4-8 pups per liter range. In certain studies, rabbits may be held in cages that are 15.25” in height. In these cages, the ears do not touch the top of cage during normal postural movements of the rabbits. 2. Environmental Enrichment, Social and Behavioral Management [Guide, pp. 52-55; 63: Ag Guide, Chapter 4] a. Enrichment i. Describe the structural elements of the environment of primary enclosures that may enhance the well-being of animals housed (e.g. resting boards, privacy areas, shelves/perches, swings, hammocks, etc.). Most rodents are housed on contact bedding, which allows expression of species-typical behaviors such as digging. Some rodent cages are equipped with elevated resting areas or “lofts” within the cage. Group-housed cats are provided with multilayered shelves for climbing and resting. Most birds are housed in large outdoor flight cages with multiple perches, swings and nest boxes. Birds housed in smaller cages have perches. Xenopus frogs have privacy areas (PVC tubes). 45 2/11 ii. Describe nonstructural provisions to encourage animals to exhibit species-typical activity patterns (e.g., exercise, gnawing, access to pens, opportunity for exploration, control over environment, foraging, denning, burrowing, nesting materials, toys/manipulanda, browsing, grazing, rooting, climbing). Mice have nesting material. Rats have paper towels for shredding, and may have Nyla-bones or wooden tongue depressors for gnawing. Rabbits have PVC pips, plastic chains or balls and hay. Pigs have sanitizable toys such as Kong toys or plastic balls, as well as brushes attached to the pen walls for rubbing and scratching their skin. Group housed cats have large multilevel shelves for climbing, as well as various toys and scratching materials. Birds housed in outdoor flight cages have opportunities for nesting and foraging. b. Social Environment [Guide, p. 64] i. Describe institutional policy or strategy for social housing of social species. The policy for social housing of social animals is incorporated into the following IACUC policy on environmental enrichment: “In compliance with Federal Animal Welfare Regulations and guidance and in consideration of the physical and social needs of research animals the IACUC requires that appropriate environmental enrichment be provided to standard animal housing unless there is scientific justification, approved by the IACUC that precludes the use of environmental enrichment materials or practices.” Most animals are group-housed if compatible. ii. If social animals are not socially housed, provide justification, as approved by the IACUC/OB. Although the default is to group-house most species when compatible, social species may be single-housed for various reasons, including: 1. Possible interference with instrumentation such as cranial implants 2. Requirement to house individually in metabolic cages 3. During post-operative recovery 4. Due to lack of appropriate caging large enough for grouphousing (rabbits). We are working on implementing group housing of female rabbits. 5. Adult male mice that are not littermates or that have been used for breeding cannot be group-housed due to excess aggression. 46 2/11 6. Adult male rabbits cannot be groups housed due to aggression; similarly, male mice of known aggressive strains e.g. SJL or Tg2576 cannot be group housed. iii. Describe steps taken with isolated or individually housed animals to compensate for the absence of other animals (e.g., interaction with humans, environmental enrichment, etc.). Single-housed rodents and rabbits are provided with nesting material (rodents) and toys (rabbits). Most single-housed rats are housed in open-topped wire-bar lidded cages, which allow them to hear and smell other rats. Rabbits are housed in cages that allow them to see, hear and smell other rabbits. c. Procedural Habituation and Training of Animals [Guide, pp. 64-65] Describe how animals are habituated to routine husbandry or experimental procedures, when possible, to assist animals to better cope with their environment by reducing stress associated with novel procedures or people. Animals arriving at UCI are required to undergo an acclimatization period prior to being used for experimentation. This allows animals to habituate to the new environment and the routine husbandry procedures. For rodents, the acclimatization period is 48 hours. For rabbits and larger animals, the acclimatization period is 72 hours. Animals used in behavioral testing paradigms typically undergo a training period to habituate them to the testing apparatus and procedure. Positive reinforcement such as food treats and positive human interactions are generally used to condition the animals to the testing environment. d. Enrichment, Social and Behavioral Management Program Review [Guide, pp. 58, 69] Describe how enrichment programs and exceptions to social housing of social species are regularly reviewed to ensure that they are beneficial to animal wellbeing and consistent with the goals of animal use. The IACUC’s environmental enrichment policy is reviewed periodically by the committee and ULAR veterinarians. Housing exceptions, including requests for single housing of social species or requests to withhold standard enrichment items, are reviewed at the time of de novo protocol review. e. Sheltered or Outdoor Housing [Guide, pp. 54-55] i. Describe the environment (e.g., barn, corral, pasture, field enclosure, flight cage, pond, or island). 47 2/11 Finches are housed in large outdoor flight cages constructed of pipe and wire mesh. The flight cages have cement floors and multiple perches. Nesting boxes and nesting material (grass and feathers) is provided. ii. Describe methods used to protect animals from weather extremes, predators, and escape (e.g., windbreaks, shelters, shaded areas, areas with forced ventilation, heat radiating structures, access to conditioned spaces, etc.). The flight cages are partially sheltered and thermostatically controlled heaters are used to protect the animals from weather extremes. Birds can shelter from wind, sun and rain. In really cold weather, birds can be confined in the sheltered portion of the flights if necessary. A double mesh ceiling protects finches from occasional raptors. iii. Describe protective or escape mechanisms for submissive animals, how access to food and water is assured, provisions for enrichment, and efforts to group compatible animals. Food and water are provided ad libitum in the flight cages, in multiple containers large enough to allow many birds to access them at the same time. The flights are large (40 feet x10 feet x 7.5 feet) and the birds are small, so subordinate individuals can easily avoid more dominant ones. Enrichment is provided by social housing and opportunities for species-typical behaviors such as nesting, breeding, allopreening, sunbathing, and foraging within the flight cages. f. Naturalistic Environments [Guide, p. 55] i. Describe types of naturalistic environments (forests, islands) and how animals are monitored for animal well-being. UCI does not perform studies that require naturalistic environments such as pastures or islands. However, the IACUC has approved a protocol that allows rats to be placed in a “naturalistic” setting that allows the animals to perform behaviors such as digging and foraging for food. The protocol allows for housing rats in 6-ft. diameter soil-filled troughs covered with wire mesh. The rats are then allowed to burrow within the soil. The animals are checked daily by laboratory personnel, water and food changed daily, and the soil changed between cohorts. The apparatus is located in existing animal facilities or within laboratory room, and proved controlled lighting and ventilation per recommendations of the Guide. 48 2/11 ii. Describe how food, water, and shelter are provided. Food is supplied in feed hoppers that are moved daily to force animals to forage for food. iii. Describe how animals are captured. Animals are hand-captured at the completion of each experiment. C. Animal Facility Management 1. Husbandry a. Food [Guide, pp. 65-67] i. List type and source of food stuffs. Mammals are fed research-grade commercial diets from either Purina Lab-Diet through Newco Distributors or Harlan Teklad. Some investigators purchase special diets that satisfy specific research protocols after review and approval by ULAR and/or IACUC, as appropriate. At the aviary, the principal food type is a commercial seed mix designed for finches and budgies purchased from Magnolia Bird Farm. Cuttlebone and oyster shell are added for calcium and minerals. Fresh food such as hard boiled hen’s egg or a mixture of cooked rice and raw peas are provided to the birds on a regular schedule. Alligators and snakes are fed a variety of food, including: chicken (purchased from local supermarkets, either cut into appropriately-sized chunks, or ground in a commercial meat grinder), commercial pellets, or whole rodents (previously euthanized by CO2 inhalation) or occasionally, to assist newly hatched or sick animals, crickets. Adult axolotls are fed pellets and brine shrimp larvae. Frogs are provided frog pellets (Nasco or Rangen, Inc) and/or live bloodworms. Zebra fish are fed either brine shrimp or Silver Cup Zebrafish Food from Aquatica Tropicals. ii. Describe storage facilities of vendors, noting temperature and vermin control measures. If more than one source, describe each. The majority of animal feed is purchased from either Purina Labdiet through Newco Distributors or Harlan Teklad. Newco is an ISO 9001:2000 certified company and a Purina Mills Certified Lab Dealer. Laboratory diets are stored in an environmentally controlled (temperature maintained at 72°F) and monitored room within in a 60,000 SF cement building. All materials are stored on pallets off the floor and feed is rotated to assure freshness, usually within a one-week period. Feed is manufactured exclusively in the Purina Mills ISO 9001:2000 Richmond, Indiana facility. No antibiotics, 49 2/11 synthetic estrogen, or other such chemicals enter the facility. Only FDA and AAFCO approved feed additives for feed nutritional uses are permitted in the facility. Both Purina Mills and Newco Distributors, Inc. have certified pest control services for their facilities. Rodent control is accomplished with liquid bait stations on the outside of the facility. Non-toxic glue boards are used in the interior of the facility as well as pheromone traps for flying insects. Further insect control is conducted by the certified pest control service utilizing Pyrethrin/Piperonyl-Butoxide when necessary. For Harlan Teklad, feed is shipped from Madison, WI to a local warehouse. Both the manufacturing plant and warehouse are ISO 9001 certified. In this warehouse, feed is stored off the ground at least 18” from the wall. The warehouse is inspected and cleaned daily. Temperature is controlled to never exceed 70°F or 55% humidity. Vermin control is accomplished through exclusion barriers, sanitation, observation, and glue boards and pheromone traps. iii. Describe bulk food storage facilities, if applicable, noting temperature and vermin control measures. Note food storage areas within the specific animal facilities are described below in Section IV.B.4.a. Physical Plant. Feed is delivered weekly. The mill dates are checked upon arrival and stored to ensure first-in first-out in feed rooms within the animal facility or a central storage building. Temperature in all of the storage facilities is maintained at 68°-72°F and floors are swept and mopped weekly. Vermin control is accomplished through exclusion barriers, immediate sanitation of any spilled product, daily observation for any sign of infestation, and use of baited spring traps. On the north campus, bird seed is stored unopened at room temperature until 3 days prior to use, at which time it is placed into a freezer for 3 days. The freezing procedure kills immature forms of moths and beetles that are frequently found in these seed mixtures, but does not compromise the nutritional value of the seed and the seed still germinates after treatment. The seed is then transferred to lined, plastic garbage cans and used immediately. iv. Describe food storage in animal rooms. Feed is placed into plastic bins with plastic liners and tight fitting lids. A label with the type of feed, mill date and date placed in the animal room is attached to the bin. v. Describe food preparation areas. N/A 50 2/11 vi. Describe how food is provided to various species (ad libitum, limited amounts, types of feeders). Food is provided daily ad lib to most rodents with the exception of animals being fasted or limit fed as part of a research protocol. Most rodent food is placed in wire bar food hoppers; feed is placed on the cage bottom for gerbils and occasionally for mice and rats. Cats are fed for a 4 hour period each day. Pigs and rabbits are fed a measured amount of diet to prevent excessive weight gain. In the aviary, food is provided on the floor of the flights, in pans or bowls that are generally set in larger containers to contain seed husks, etc. Cuttlebone holders are mounted on the wall of flights. In bird cages, food is provided in bowls on the floor of the cage, and cuttlebone is mounted on the side of the cage. vii. Describe special food quality control procedures including procedures for rotating stock, monitoring milling dates, nutritional quality, bio-load, chemical contaminants, etc. Food is purchased from established reputable sources. Responsibility is placed on the manufacturer and vendors with regard to nutritional quality, bio-load or possibility of chemical contaminants. The mill date is checked upon receipt. Food with the oldest milling date is used first. No food in excess of six months from the milling date is used. As the seed is not milled and the seed coat is intact, the shelf life of commercial bird seed is quite long when stored appropriately. Bags are dated upon receipt and older seed is used before recently acquired seed. It is generally used within six months of purchase. Seed that has become wet or is moldy is discarded. b. Drinking Water [Guide, pp. 67-68] i. Describe the water source, treatment or purification process, and how it is provided to the animals (e.g., bowls, bottles with sipper tubes, automatic watering, troughs, ponds, streams, etc.). Water is obtained from the city of Irvine. It is potable water by state health standards. No additional treatment or purification is provided, unless required by a protocol or specially conditioned animals. Water bottles are used in all facilities. They are monitored daily, replaced as required and sanitized at least once a week. Immunocompromised animals and barrier mice receive water that has been autoclaved. Automatic watering devices are used for pigs and most of the rabbits. The automatic water systems employ particulate filters (Edstrom) in Med Sci A. At UCIMC Bdg. 55, the automatic water system utilizes UV light purification. At the aviary, water is provided in plastic containers or glass bowls (in small cages only); 51 2/11 all aviary water containers are currently hand-washed daily in hot water and the water is changed daily. ii. Describe methods of quality control, including monitoring for contaminants. The water within University buildings is sampled at least once a month by Facilities Management and tested for alkalinity, iron content, and amount of total dissolved solids contained in the water. The Irvine Ranch Water District publishes a summary of water quality testing annually and this document is reviewed by ULAR veterinarians. In addition, ULAR performs in-house analysis of drinking water for coliforms and total bacterial counts quarterly. iii. If automatic water delivery systems are used, describe how they are maintained and sanitized. The water delivered into the automatic watering system is disinfected via ultraviolet light. The automatic watering systems are flushed monthly. For rabbits on automatic water system, the lixits are sanitized along with the animal racks in our mechanical rack washers. For pigs on automatic watering systems, the lixits are sprayed with a high pressure hose during pen cleaning. c. Bedding and Nesting Materials [Guide, pp. 68-69] i. Describe type(s) and how used for various species. The primary material used for contact rodent bedding is ground corncobs purchased from Harlan Teklad. Sani-Chips, a hardwood product or Alpha-dri, a paper product, is used for some rodents. All rodent cages receive a measured amount of bedding in the clean cage and the bedding is changed only when the cage is sanitized. Sani-Chips are used for cat litter boxes and hardwood shavings are used as bedding in the pig runs, and these are changed daily. Recycled newspaper is used for the reptiles and finches. Noncontact paper liners are used for rabbits and are changed three times per week. ii. Describe bulk bedding storage facilities, if applicable, including vermin control measures. Note bedding storage areas within the specific animal facilities are described below in Section IV.B.4.a. Bedding is stored in the storage room of each facility or in a central storage building. The room is cleaned weekly and vermin control is accomplished through exclusion barriers, immediate sanitation of any spilled product, daily observation for any sign of infestation, and use of baited spring traps. 52 2/11 iii. Describe quality control procedures, including monitoring for contaminants. Bedding is rotated to prevent long term storage of a given bag. Complete technical specifications and the quality control measures for contaminants of the bedding products used are available from the manufacturer. No local monitoring for contaminants is performed. d. Miscellaneous Animal Care and Use Equipment i. Describe motorized vehicles and other equipment (e.g., trailers) used for transporting animals, noting the type and how the cargo compartment is environmentally controlled, if applicable. ULAR maintains three climate controlled vehicles for transport of animals between animal facilities and nearby research institutes. ULAR will authorize individual researchers to transport small numbers of rodents between the main campus and the medical center or nearby research institutes. Prior to authorizing a shipment, a ULAR veterinarian reviews concerns related to transportation. Such animals must be contained within a commercial (filtered) transport box or a sealed microisolator cage. The transport box or cage must be in a ventilated vehicle compartment (i.e. not a trunk). ii. Describe other animal care related equipment used in the animal care program (e.g., specialized equipment for exercise or enrichment, high pressure sprayers, vacuum cleaners, tractors, trailers, spreaders, etc.). Floor scrubbers and high pressure sprayers are available. e. Sanitation [Guide, pp. 69-73] i. Bedding/Substrate Change 1) Describe frequency of contact and non-contact bedding change for each species and enclosure type (solid-bottom or suspended) or pen. SPECIES Birds (multiple spp.) Cat Gerbil Guinea pig Hamsters Mice CAGE TYPE Flight and wire cages Runs/room Solid bottom Solid bottom Solid bottom Solid bottom 53 FREQUENCY 2-3x weekly Litter box changed daily 1x weekly 2-3x weekly 1-2x weekly 1-2x weekly (static) Every 7-14 days 2/11 Pig Rabbit Rat Snakes Runs Suspended Solid bottom Glass & plastic aquaria (ventilated cages) Daily 3x weekly 1-2x weekly Weekly 2) Describe any IACUC/OB-approved exceptions to frequencies recommended in the Guide or applicable regulations and the criteria used to justify those exceptions. Individually ventilated mouse cages are normally changed once every two weeks. If the cage is found to be excessively dirty or wet before this time, it will be spot changed. Wire bars are changed out once every month and filter tops are changed once every three months. We have performed intercage ammonia, moisture, and bacteria testing to show that during this time, the intercage micro-environment does not change appreciatively during this time. 3) Note the location where soiled bedding is removed from the cages/enclosures and where clean bedding is placed into the cages/enclosures. Soiled bedding is removed in the “dirty” side of the cage washer area; soiled bedding is emptied out of cages and pans into waste receptacles. Fresh bedding is added to clean cages on the “clean” side of this area. At the aviary, cages are changed in place on the shelf where the bird resides to minimize disturbance to the birds. ii. Cleaning and Disinfection of the Micro- and Macro-Environments Describe the washing/sanitizing frequency, and methods used in the Appendix, “Cleaning and Disinfection of the Micro- and MacroEnvironment.” 1) Describe any IACUC/OB-approved exceptions to the Guide (or applicable regulations) recommended sanitization intervals. Individually ventilated mouse cages are normally changed once every two weeks. If the cage is found to be excessively dirty or wet before this time, it will be spot changed. Wire bars are changed out once every month and filter tops are changed once every three months. We have performed intracage ammonia, moisture, and bacteria testing. Results indicated that during this time the intracage micro-environment does not change appreciatively. 2) Assessing the Effectiveness of Sanitation and Mechanical Washer Function 54 2/11 a) Describe how the effectiveness of sanitization procedures is monitored (e.g., water temperature monitoring, microbiological monitoring, visual inspections, etc.). Water temperature is monitored by indicator tags, heat sensitive tape and chart recorders for each cage/rack washer. The heat sensitive tape is used in each washer daily and this record is maintained in the cage wash area. Additionally, the automatic soap dispensers in the cage/rack washer area are serviced and monitored quarterly. New cage washers will not complete the wash cycle if rinse temperature fails to meet 180oF. Due to insufficient steam delivery to several older buildings, several washers do not reliably attain 180oF during the rinse cycle. To achieve adequate sanitation, these units are supplied with chlorinated detergent (GNRF, Bonney Center, and McGaugh Hall). Luminometer testing of clean cage components are performed monthly from each cage washer. Autoclaves are monitored by means of continuous recording charts, which are archived in the animal facilities manager’s office, and by the use of indicator tape placed on material in each load. Further, microbiological spore testing is performed monthly on all ULAR autoclaves. Water is monitored quarterly by analyzing water samples taken at the bottling source for microorganisms growth on agar plates. b) Describe preventive maintenance programs for mechanical washers. Tunnel and Rack Washer Maintenance: Daily checks and cleaning of spray header jets and stainless steel filter screens before start of operations. At the end of operation, drain cycle is initiated to empty re-circulating tanks. Weekly de-scaling cycle is activated to prevent excessive build up of hard water scale deposits. f. Waste Disposal [Guide, p. 73-74] Describe the handling, storage, method and frequency of disposal, and final disposal location for each of the following: i. Soiled bedding and refuse In the Gillespie and McGaugh facilities, cages are dumped into a down-drafted Garbel-style disposal adjacent to the tunnel washer, with the dirty bedding being disposed of in the sanitary sewer system. In the Bio Sci III facility, the cages are dumped in a vacuum 55 2/11 removal system, with the dirty bedding going into an enclosed dumpster. In Med Sci A and UCIMC bldg 55, cages are dumped into plastic lined garbage cans within a HEPA-filtered dump station. In the Bonney Center the cages are dumped in the open. Bedding is then transported to a dumpster located outside the building. Dumpsters are emptied as needed and waste transported to a sanitary landfill by an outside contractor. ii. Animal carcasses With the exception of pigs, all animal carcasses, including biohazardous and chemically contaminated, are handled as Pathological Waste. They are placed within plastic bags, sealed, and placed in a designated freezer or cold-box located in each animal facility. Carcasses are then removed by EH&S and disposed of by a certified incineration company. Pig carcasses are shipped to a rendering plant. iii. Hazardous wastes - infectious, toxic, radioactive, sharps and glass Dirty biohazardous rodent cages with bedding are autoclaved and then the dirty bedding is bagged and stored in a cold box. Disposal is handled by EH&S utilizing a local, certified biohazard waste vendor. Chemically contaminated bedding is handled as pathological waste and is incinerated. Contaminated carcasses, cages with dirty bedding, and other supplies classified as ABSL-3 are bagged within the ABSL3 holding room or laboratory and then decontaminated by a certified autoclave in Hewitt Hall. Radioactive animal carcasses and bedding material are handled as radioactive wastes under the direction of EH&S and collected by an EH&S technician. They are then stored frozen at EH&S facilities until radioactivity has decayed sufficiently for disposal as a nonradioactive waste, or until packed in an approved manner for transport by an outside contractor to an NRC licensed disposal facility. g. Pest Control [Guide, p. 74] i. Describe the program for controlling pests (insects, rodents, predators, etc.) noting the control agent(s) used, where applied, and who oversees the program and applies the agent(s). Include a description of natural predators (e.g., barn cats) or guard animals (e.g., dogs, donkeys) used for pest and predator control, if applicable. Major emphasis for controlling vermin is focused on good sanitary practices, eliminating breeding and refuge sites, and maintaining facilities to prevent vermin entry. Facilities are monitored for wall, ceiling or floor cracks. Any damaged area is immediately reported, 56 2/11 and a work order is submitted to building management for repair. Doors are equipped with self-sealing sweeps. University Facilities Management maintains baited rodent stations on the exterior of buildings and within landscape areas to control wild rodents. When a feral rodent is identified within an animal facility, attempts are made to capture the escaped animal. If these efforts are unsuccessful, baited spring traps and/or glue traps may be employed by ULAR personnel. We have identified rodent issues, either feral or wild, in laboratory areas of several buildings. Where identified, various types of live traps and/or baited spring traps have been used, placed by research staff and/or facility personnel. Occasional infestations of crickets have been noted in several facilities. Control is through the use of sticky traps and chemically treating exposed drains with “Fly Away”, which is a surfactant without any pesticides. ii. Note how animal users are informed of pesticide use and how animal users may opt out of such use in specific areas. No pesticides are used. h. Emergency, Weekend and Holiday Care [Guide, pp. 74-75] i. Describe procedures for providing weekend and holiday care. Indicate who (e.g., regular animal care staff, students, part-time staff, etc.) provides and oversees care and what procedures are performed. Indicate qualifications of weekend/holiday staff if not regular staff. ULAR regular husbandry staff share responsibilities for weekend care of the animals on a rotating weekend basis. All animals are observed on a daily basis, checking animal health and cage condition and ensuring the availability of food and water. On weekends, rodent and rabbit cages are changed or cleaned as needed. Cat rooms and pig runs are cleaned daily, including weekends. For investigator maintained animals, the investigator’s staff is responsible for providing daily care and feeding. The IACUC requires that a daily log be maintained on all animal housing rooms, including investigator maintained rooms to ensure all animals are checked daily. ii. Describe procedures for contacting responsible animal care and/or veterinary personnel in case of an emergency. ULAR veterinarians and ULAR supervisors provide after-hours coverage on a rotating basis. Personnel can be reached at all times by calling a phone tree number, which will direct the caller to on-call staff. Signage with instructions for contacting personnel for after hour emergency care is posted in all animal facilities. 57 2/11 2. Population Management [Guide, pp. 75-77] a. Identification Describe animal identification methods for each species (e.g., microchips, cage/tank cards, collars, leg bands, tattoo, ear tags, brands, etc.). Cards are placed on cage fronts in metal and plastic card holders for all animals. For purchased animals, information contained on the cage cards includes the following: investigator’s name, protocol (IACUC) number, laboratory contact name and phone number, vendor name, Purchase Order number, species, strain, date of arrival, the animal’s DOB or weight upon arrival, and sex. Some of this information will not be present on the cage card for animals that were bred at UCI. For rodents, typically there will be only one cage card per cage. If an investigator wants the individual rodent ID’d, it is their responsibility to perform this after review and approval of method of identification in the IACUC protocol. Various means are utilized, including ear punches, ear tags, toe or tail tattoos, microchips, and distal toe clipping. At the aviary, each bird wears a uniquely numbered leg band; most birds also have unique color band combinations. For caged birds, bird identification is listed on cage cards, which are placed on the front of the bird cages. The cage card also lists the purpose of housing the bird in a cage (e.g., the name of a particular experiment), and the birds’ sex (when known). In addition to cage cards, rabbits, cats, and pigs have either a tattoo ID number or an ear tag for identification. b. Record Keeping Describe procedure(s) for maintaining individual records on animals. Identify the species for which individual records are maintained, individuals (titles, not necessarily names) responsible for maintaining the records, and where they are maintained and how veterinary and IACUC/OB access is assured. All incoming shipments of animals are entered into an in-house database. ULAR maintains individual records for cats and on other individual animals when illness occurs or if individual medical therapy is provided by Veterinary Services staff. Such records are maintained by Veterinary Services, either as a hard copy or in an electronic database. Investigators are responsible for maintaining individual procedure records, including surgery, anesthesia, and post-operative monitoring. These records are to be kept on file in their laboratories, but must be available to ULAR and IACUC on request. Such records are periodically reviewed by the IACUC during laboratory visits. c. Breeding, Genetics and Nomenclature i. Describe the program for advising investigators on the selection of animals based on genetic characteristics. 58 2/11 Investigators may request assistance from the campus veterinarian on selection of particularly suitable genetic stocks or may be required to do so by the IACUC. Relevant publications are maintained in the ULAR offices and are available to investigators. ii. Describe the program for advising investigators on using standardized nomenclature to ensure proper reporting of the identification of the research animals with regard to both the strain and substrain or the genetic background of all animals used in a study. Investigators that propose to create or maintain breeding programs (i.e.: genetically modified rodents) must describe their record keeping system, nomenclature and method of identifying animals in the IACUC protocol. Investigators seeking guidance on proper nomenclature for identifying genetically modified strains are directed to information hosted on the Jackson Laboratory web site. Investigators may also consult with ULAR veterinarians and personnel in the Transgenic Mouse Facility. iii. For newly generated genotypes, describe how new phenotypes that negatively impact well-being will be monitored, managed and reported to the IACUC/OB in a manner to ensure the animals’ health and well-being. As part of the IACUC protocol, the researcher is asked to discuss the phenotypic abnormalities that may be present in the animals. This explanation must include possible clinical signs and symptoms, monitoring parameters, management plan, frequency and documentation of monitoring, and euthanasia criteria. These parameters may vary depending on the genotype, severity of phenotype, and nature of the research. The IACUC then considers whether modifications to the proposed plan need to be implemented in order to ensure the animals’ health and well-being before approving the protocol. III. Veterinary Care [Guide, pp. 105-132] Note: Complete each section, including, where applicable, procedures performed in farm settings, field studies, aquatic environments, etc. A. Animal Procurement and Transportation [Guide, pp. 106-109; Ag Guide, pp. 8; 45; 51-57] 1. Animal Procurement Describe the method for evaluating the quality of animals supplied to the institution (e.g., from commercial vendors, other institutions, etc.). To ensure consistent quality of animals, acquisition (purchase or transfer) of research animals is coordinated by the ULAR office. Most of the rodents used at UCI are purchased from commercial vendors that breed animals 59 2/11 specifically for use in research and teaching. These vendors perform regular examinations of their production stock, including testing the animals for the common infectious agents of each species. Test results are reviewed by ULAR regularly and vendors that consistently meet ULAR's standards are placed on an approved vendor list. Animals from these approved vendors can be introduced directly into UCI animal facilities. Rodents from unapproved sources, such as non-commercial vendors, other universities, government agencies, and commercial vendors with inadequate health assurance information are placed directly into one of two quarantine rooms located in McGaugh Hall. Prior to accepting rodents into quarantine, the health history from the sending institution is reviewed. If no health concerns are identified, the animals are accepted. If there are health concerns, the animals must be rederived either at the sending institution or here at UCI. If the rederivation will be performed at UCI, the animals will be brought into an isolation facility in Steinhaus Hall. 2. Transportation of Animals Describe how animals are transported between outside sources and the institution and within the institution, including loading, unloading, level of biosecurity, immune status and specific pathogen status (consider all species, including aquatic and semi-aquatic species). Mammals are transported to UCI directly by the vendor, a commercial carrier selected by the vendor, or by USDA-licensed transporters. The majority of amphibians, reptiles and avian species (i.e.: chicks) are sent by express mail to the university. Some reptiles (alligators from Louisiana) are transported by private vehicles driven by UCI research personnel after obtaining special permission from ULAR and/or the IACUC. Upon delivery, the shipment is inspected by ULAR personnel for any visible external damage and brought into the facility. If the animals are contained within a shipping box, the outside of the box is sprayed with facility approved disinfectant in the receiving area before unpacking the animals in the animal holding room. ULAR maintains three climate-controlled vehicles for transport of animals and supplies between animal facilities and nearby research institutes. All cages, whether being transported by ULAR or research staff, must be covered when taken out of the animal facility so other personnel cannot see that an animal is being transported. Infrequently, ULAR will authorize individual researchers to transport small numbers of rodents between the main campus and the medical center or nearby research institutes. Prior to authorizing a shipment, a ULAR veterinarian reviews concerns related to transportation. Such animals must be contained within a commercial (filtered) transport box or a sealed microisolator cage. The transport box or cage must be in a ventilated vehicle compartment (i.e. not a trunk). B. Preventive Medicine 60 2/11 1. Animal Biosecurity [Guide, pp. 109-110] a. Describe methods used to monitor for known or unknown infectious agents. A rodent sentinel program is maintained by ULAR. Serological testing, parasitology, and when indicated from gross necropsy, histological examination of tissues are performed on sentinel rodents from each room quarterly. Sentinel mice and rats are bred within the sentinel cage or purchased by ULAR and are placed in all long-term, non-biohazard rodent rooms. When cages are changed, soiled bedding from colony animals is placed into the sentinel animal cages. Animals are necropsied at UCI and sera submitted for testing at RADIL. Additional testing includes examination of the pelt for ectoparasites, and gross examination of cecal contents and perianal tape test to identify enteric parasites. b. Describe methods used to control, contain, or eliminate infectious agents. Animals suspected of, or infected with unwanted infectious agents are promptly removed via euthanasia or movement into the isolation facility in Steinhaus Hall, or the housing room is placed under quarantine to reduce exposure and possible disease spread to the general animal facility population. Veterinary Services notifies affected research personnel immediately and provides necessary training. Appropriate isolation equipment (gowns, shoe covers, etc.) is provided immediately outside the quarantined housing room. Depending on the infectious agent, additional testing may be instituted to determine the extent of the problem and a treatment or eradication plan developed and implemented after discussion with the affected researcher(s). 2. Quarantine and Stabilization [Guide, pp. 110-111] a. Describe the initial animal evaluation procedures for each species. Animals are received at the designated receiving area at each animal facility. Rodent shipping containers are sprayed with a disinfectant prior to opening each box. As animals are transferred from shipping containers into holding cages, technicians observe for abnormalities and overt signs of disease. Any abnormalities are brought to the attention of the veterinary staff and the decision to accept or reject the animal is made at that time. b. Describe quarantine procedures for each species that are purpose bred. All rodents originating from unapproved sources must be quarantined prior to release into the general population. Animals typically remain in quarantine for 7-8 weeks. The rodents are placed on fenbendazole medicated feed for endoparasites and treated topically with selamectin 61 2/11 for ectoparasites while in quarantine. Sentinels that have been placed with the shipment undergo testing (serology and parasitology) for a number of rodent infectious agents. Alternatively, the animals may be PCR tested immediately upon arrival via fecal pellets, oral and pelt swabs. Animals testing positive for excluded agents are either sacrificed or rederived prior to being released to animal facilities on campus. Bringing rodents directly into a researcher’s laboratory is allowed if the animals will be euthanized immediately upon arrival and the researcher has both IACUC and ULAR approval. The purchase of larger species such as cats, rabbits and swine is from Class A vendors and to a lesser degree from other universities. To limit the possibility of introduction and spread of infectious disease, the number of approved vendors is limited and normally animals from only one vendor are allowed in an animal facility at any one time. These animals are not normally quarantined. c. Describe the quarantine facilities. In your description explain any special measures used for quarantine/conditioning of each random source (not bred and raised specifically for research) species used. There are two rooms in McGaugh Hall that are used for quarantine of mice. Each room is managed as all-in/all-out. When rats arrive that need quarantine, a room is assigned for this. Random source species used at UCI include several avian species (finches, parakeets, chickens), fish (goldfish, zebra fish), amphibians (axolotls, Xenopus spp., Rana spp., Bufo marinus) and reptiles (snakes, turtles and alligators). Due to space limitations, separate quarantine for each of these species is not possible. However, the number of vendors providing these species is generally limited to one or two sources, and whenever possible one group of animals is depopulated prior to introduction of the next group. In addition, for birds, newly purchased animals are separated for several weeks from resident birds to allow clinical assessment for signs of disease. New zebra fish being brought in are kept in isolation tanks, and after eggs are produced, the eggs are disinfected and then allowed into the general tanks. d. Describe the required/recommended stabilization period for each species. Rodents and non-mammalian vertebrates: Rodents and nonmammalian vertebrates such as birds, amphibians and reptiles must have a minimum acclimation period of 48 hours before undergoing any survival procedures. For terminal procedures, an acclimation period is not required. Other mammals: Non-rodent mammals must have a minimum acclimation period of 72 hours before undergoing survival procedures. In addition, before undergoing any research procedures, animals must appear healthy with normal appetites and normal other bodily functions. For terminal research procedures, an acclimation period of 72 hours is recommended to ensure the validity of research 62 2/11 results. For terminal training procedures, an acclimation period is not required. e. Describe the program for the separation of animals by species, source, and health status. If the animals in different status are not maintained separately, describe circumstances in which mixing occurs and explain the rationale for mixing. Animals are separated by species by housing different species in separate rooms or in separate cubicles within a room. In rodent rooms, animals from different sources are likely to be in the same room. For larger species, normally only one source for each species is used. The IACUC has approved an exception to this in Irvine Hall where rats and mice are maintained in a single animal holding room. (See 3.b. below) 3. Separation by Health Status and Species [Guide, pp. 111-112] a. Describe isolation procedures and related facilities for animals. Animals suspected of, or infected with unwanted infectious agents are promptly removed via euthanasia or movement into the isolation facility in Steinhaus Hall, or the housing room placed under quarantine to reduce exposure and possible disease spread to the general animal facility population. Veterinary Services notifies affected research personnel immediately and provides necessary training. Appropriate isolation equipment (gowns, shoe covers, etc.) is provided immediately outside the quarantined housing room. Depending on the infectious agent, additional testing may be instituted to determine the extent of the problem and a treatment or eradication plan developed and implemented after discussion with the affected researcher(s). b. Describe situations where multiple species may be housed in the same room, area, or enclosure. Irvine Hall has one animal housing room with both rats and mice maintained in the same animal holding room. The researchers in this building routinely do studies requiring the close proximity of animals to their labs. The mice in this room are maintained within positively ventilated cages with the cage exhaust directly ventilated out of the room, thus minimizing exposure to rat pheromones. 4. Surveillance, Diagnosis, Treatment and Control of Disease [Guide, pp. 112-113] a. Describe 1) the procedure(s) for daily observation of animals for illness or abnormal behavior, 2) the observer’s training for this responsibility, and 3) method for reporting observations (written or verbal). Include a description of the method for ensuring that reported cases are appropriately managed in a timely manner. 63 2/11 All animals are observed daily by ULAR husbandry staff or trained research staff. ULAR employees are instructed on the basic methodology for the care and maintenance of animals using on-the-job training. Abnormal animal appearance and behavior is discussed at least once per year at the general staff training sessions. The animal room husbandry log contains a daily check box for observation of health of animals. For investigator maintained animals, all research personnel are trained by the Principal Investigator (PI) and ULAR to recognize sick or injured animals and to report injuries to the PI and to vet services. All animals, including investigator maintained, are checked every day, including weekends and holidays. Animals found showing signs of injury, illness or other abnormalities are reported to Veterinary Services via a written Animal Health Report (AHR). This AHR is faxed to Veterinary Services personnel. Urgent health problems are also reported to Veterinary Services by phone. b. Describe the methods of communication between the animal care staff/veterinarians and the researcher(s). Cages/animals with a health issue are flagged with a special Veterinary Observation cage card. The Animal Health Report (AHR) with initial observations and any updates is kept in the animal housing room. The research staff is contacted via email, in-person, or phone any time before treatment is initiated or if some action is required from the research staff, such as the animal needs to euthanized. The research staff is contacted by the animal husbandry technician whenever an animal is found dead or a cage is identified that needs attention, such as when a litter is present that needs to be weaned. c. Describe the procedure for providing veterinary medical care to ill animals and note who is contacted and the method of communicating (written or verbal) information to the veterinarian regarding sick animals. Animals are examined by an AHT or veterinarian and notations are made on the AHR. For routine cases, (fight wounds on a mouse for example), treatment maybe initiated by the AHT without consultation with a veterinarian. Normally the research staff will be contacted to discuss options prior to executing therapy. For all non-routine or more involved cases, a veterinarian will be directly involved in the diagnosis and treatment. d. Describe the preventive medicine and health management/ monitoring programs (e.g., physical examination, TB testing, vaccination, hoof/nail trimming, teeth cleaning/floating, vendor surveillance, use of sentinel animals, etc.) for each species. 64 2/11 All animals’ health is checked daily; any abnormalities are brought to the attention of the veterinary staff. No TB testing or vaccination is performed on any animals. Long-term rabbits have their nails trimmed at least every 4 months. C. Clinical Care and Management [Guide, pp. 113-115] 1. Emergency Care [Guide, p. 114] a. Describe the procedures to ensure that emergency care is continuously available for animals during and outside of regular work hours. In the event of an animal health emergency during regular work hours, the veterinarian on-duty is available by contacting the animal facilities offices or ULAR office staff. ULAR veterinarians provide after-hours coverage on a rotating basis. Personnel can be reached at all times by calling a phone tree number, which will direct them to the person that is on call. Appropriate signage for after hour emergency care is posted in the animal facility as well as ULAR’s website and during research animal training. b. Describe the authority of the Attending Veterinarian or his/her designee relative to the emergency treatment of animals in the program. In the event of an animal emergency, the Attending Veterinarian (AV) has ultimate authority. If possible, the AV, or his designee, will attempt to contact the research staff before initiating any treatment or implementing other measures. But in instances where time does not permit this or the research staff can not be reached, or if there is disagreement between the AV and the research staff, the AV will implement the course of action that he/she sees fit. The AV is authorized to enter any place within the jurisdiction of UCI to conduct on-site inspections of animal facilities and animals to ensure that animal husbandry, sanitation practices, and animal health and research techniques are in compliance with relevant federal, state, local, and institutional requirements. The AV is authorized to halt any animal activity if the safety or welfare of an animal is at risk or if the work being performed on a project is not in accordance with the approved protocol. 2. Clinical Record Keeping [Guide, p. 115] Describe the procedure for maintaining medical records and documenting treatment of ill animals including: clinical laboratory findings, diagnoses, treatments, medical progress records, etc. Identify individual(s) (titles, not necessarily names) responsible for maintaining such records and identify where the records are maintained and who has access to the records. Describe the role of the Attending Veterinarian in record keeping. 65 2/11 All clinical observations, diagnostics, updates, and treatments are documented on the AHR, which are maintained in the housing room while the case is open. Information is also entered into an electronic database which assists in the management of open cases and makes the information readily available to all AHTs and veterinarians. Once a case is resolved, the paper copy of the AHR is destroyed after all pertinent information is entered electronically. 3. Diagnostic Resources. Describe available diagnostic methods used in the program including: a. In-house diagnostic laboratory capabilities. Procedure rooms are available to obtain samples for evaluation in each of the major facilities. A procedure room in Med Sci A is equipped with scales, microscopes, centrifuges, incubator, and biosafety hood for performing or prepping samples for routine clinical laboratory testing, parasitology and gross pathology. b. Commercially provided diagnostic laboratory services. UCI utilizes commercial laboratories for diagnostic services other than parasitology and gross pathology. Bacteriological, hematological, histopathological, serum chemistry and serological assays are performed by commercial laboratories, with RADIL utilized most often. c. Necropsy facilities and histopathology capabilities. The necropsy room at Med. Sci. A, room 113D is used for most necropsies performed by ULAR personnel. Necropsy rooms are also available in the Gillespie Neurosciences Research Facility, Bio Sci III, and McGaugh Hall. All of these necropsies rooms are equipped with down draft and/or biosafety cabinets. Gross necropsies are performed as indicated by clinical presentation, or when requested by an investigator. d. Radiology and other imaging capabilities. Dedicated radiology facilities and equipment for animal diagnostic workups do not exist. If an animal is in need of diagnostic radiographs, several options exist, including using one of several research radiographs, utilizing the services of a local small animal veterinary hospital, treat the animal as best as possible, or having the animal humanely euthanized. 4. Drug Storage and Control a. Describe the purchase and storage of controlled and non-controlled drugs. 66 2/11 The IACUC encourages the use of pharmaceutical grade drugs when feasible and requires justification for the use of non-pharmaceutical drugs. For purchase of non-controlled drugs, a requisition is normally made through the appropriate department’s financial office. For controlled drugs, each lab must first have a Controlled Drug Usage Authorization (CSUA), which is administered by EH&S. The purchases of controlled drugs are handled by the student pharmacist at UCI. Controlled substances must be stored separately from other drugs and supplies in a locked cabinet. b. Describe record keeping procedures for controlled substances. Use of controlled drugs must be documented on a controlled substance usage log to record the agent, date used, the amount used, the animal or project identification, and the amount remaining. An inventory of controlled substances and audit of records and storage is performed annually by the EH&S Controlled Substances Coordinator. D. Surgery [Guide, pp. 115-123] 1. Pre-Surgical Planning [Guide, p. 124] Describe the process(es) used to ensure adequate pre-surgical planning, including: identifying personnel; locating equipment, supplies, veterinary involvement for selecting analgesic and anesthetic agents and facilities; planning; and pre- and postoperative care. All personnel listed on animal use protocols must complete a training program before ULAR will authorize them access to the animal facility. When they register for this training, they identify whether they will be performing surgery. If so, they need to complete the “Pain and Distress” online module through the CITI program (Collaborative Institutional Training Initiative), as well as the “Working with Live Animals in Research” module, and the module(s) specific to the species with which they will be working. Required researcher training also includes a UCI-specific classroom session presented by a ULAR veterinarian, which includes information on pain and distress. During the classroom session, the trainer discusses the availability of further training on specific procedures, including surgery and peri-operative care. The ULAR web site informs researchers that the veterinarians are available for consultation and training on animal research procedures including surgery. The ULAR and IACUC web sites provide guidelines for surgery, including requirements for training, facilities, aseptic surgery, surgical techniques, anesthetic monitoring, pre- and post-operative care and surgical records. The IACUC Application to Use Animals IACUC contains instructions that “a ULAR veterinarian should be consulted in the planning of procedures involving potential pain and/or distress”. The Application to Use Animals contains sections on pre- and post-procedural monitoring and care. 67 2/11 All IACUC protocols are pre-reviewed by a veterinarian before they are submitted for IACUC review. If the descriptions of pre-surgical planning, or peri-operative care are inadequate, the veterinarian sends comments to the IACUC administrator, who then notifies the lead researcher, so that the sections can be revised before the protocol is reviewed by the committee. Recommendations may include specific changes to the surgical procedures or peri-operative care, or may include a requirement to meet with a UCI veterinarian to discuss the project in more detail before resubmitting a revised protocol form. 2. Surgical Facilities [Guide, p. 116] a. List building name(s) and room number(s) or other locations (coded, if confidential) where surgical procedures are performed. Include areas where surgical procedures are conducted in agricultural species. Indicate the type of species, nature of procedure (major/minor/emergency; survival and nonsurvival, etc.). Indicate for each surgical area if the use is heavy (daily), moderate (weekly), or light. Building Beckman Laser Beckman Laser Bio Sci III Bonney GNRF Hewitt Irvine Hall McGaugh Room(s) Species Nature Usage 418 Maj, Survival Mod Min, Survival Maj, Survival Maj, Survival Maj, Survival Maj, Survival Min, Survival Maj, Survival Mod Mod Mod Heavy Mod Mod Mod McGaugh McGaugh 2113 B324, B365, 1432 Maj, Survival Maj, Survival Mod Mod Med Sci A 113C Rabbits Hamsters, mice Mice Rats Mice, rats Mice, rats Rats Rats Mice, Gerbils Mice, rats Rabbits, cats, swine Med Sci B Med Sci B 126 162 Swine Mice, rats Med Sci C Med Sci C Med Sci C 256 268 362 Med Sci D Med Sci Annex 404 Cats, rats Frogs Mice, rats Cats, guinea pigs 1041 Mice 120, 306 106, 126, 312, 332 212, 216 131, 210, 211, 228 106, 112 192 B209, B305 68 Maj, Survival, emergency Maj, nonsurvival Maj, Survival Maj, nonsurvival Maj, Survival Maj, Survival Light Light Light Mod Mod Light Maj, Survival Mod Maj, Survival Mod 2/11 Med Surge II Med Surge II 336, 359 Mice, rats 318 Cats Fish, axolotls, frogs Nat Sci II 4300, 4313, 4410 UCIMC 20 UCIMC UCIMC 82 38, 66, 76 Swine Swine, rabbits Mice, rats Maj, Survival Maj, nonsurvival Mod Maj, Survival Maj, nonsurvival Mod Maj, Survival Maj, Survival Heavy Mod Mod Mod b. List the major surgical support equipment available at each location where survival or nonsurvival surgery is performed (e.g., gas anesthesia machines, respirators, etc.). Building Beckman Laser Beckman Laser Bio Sci III Bonney GNRF Hewitt Irvine Hall McGaugh McGaugh McGaugh Med Sci A Med Sci B Med Sci B Med Sci C Med Sci C Med Sci C Med Sci D Med Sci Annex Med Surge II Med Surge II Nat Sci II UCIMC UCIMC UCIMC c. Room(s) 418 306, 308 106, 126, 312, 332 212, 216 131, 210, 211, 228 106, 112 192 B209, B305 2113 B324, B365, 1432 113C 126 162 256 268 362 404 1041 336, 359 318 4300, 4313, 4410 20 82 38, 66, 76 Gas Anesthesia Available? Yes No Yes Yes Yes Yes No No No Yes Yes Yes No Yes No No Yes Yes No Yes No Yes Yes Yes Ventilator & Monitor Available? Yes No No No No No No No No No Yes Yes No Yes No No Yes No No No No Yes Yes No Describe any specialized considerations for designation of surgical areas (e.g., rodents, aquatics, farm animals, etc.). 69 2/11 N/A 3. Surgical Procedures [Guide, pp. 117-118] a. Describe the criteria used to differentiate major from minor survival surgery, including classification for certain procedures (e.g., laparoscopic technique, etc.). Major survival surgery: A surgical procedure that penetrates and exposes a body cavity or produces substantial impairment of physical or physiologic functions. Examples include: laparotomy, thoracotomy, craniotomy, orthopedic procedures, and limb amputation. Minor survival surgery: A surgical procedure that does not expose a body cavity and causes little or no physical impairment. Examples include wound suturing, peripheral vessel cannulation, and placement of subcutaneous implants. b. How is non-survival surgery defined? Survival surgery: A surgical procedure from which an animal is expected to recover from anesthesia. Non-survival (Terminal) surgery: A surgical procedure from which an animal is euthanatized before recovery from anesthesia. 4. Aseptic Technique [Guide, pp. 118-119] a. Describe procedures, equipment, and protective clothing used for aseptic surgery. Include patient and surgeon preparation. Survival surgery must be performed using aseptic techniques. The IACUC has developed a Surgery Policy and Guidelines and ULAR has posted aseptic surgery guidelines, including performing the surgery in a dedicated space, removal of hair, cleaning and disinfecting the surgical site, usage of sterile gloves and instruments, and adherence to appropriate technique during surgery. The investigator is responsible for supervising the surgical procedure and providing adequate support staff, instruments, supplies and drugs. Major survival surgical procedures in large animal species are conducted in dedicated surgical suites intended for that purpose, maintained and operated under aseptic conditions. Surgery on rodents does not require a dedicated surgical suite. ULAR Veterinary Services provides training in aseptic surgical technique upon request and as needed. b. Describe methods used to sterilize instruments and protective clothing. Indicate how effectiveness of sterilization is monitored and, if applicable, any approved alternate methods for instrument re-sterilization between serial surgeries. If used, include a description of approved liquid sterilants and instrument exposure time(s) required for each. 70 2/11 Methods used to sterilize instruments and other equipment include autoclaving, gas sterilization, or use of a hot-bead device for sterilizing instruments in between multiple rodent surgeries. Autoclaves are available in or near each large animal surgical suite (Med. Sci. A, Beckman Laser, and UCIMC Bldg. 55) as well as multiple other locations. Temperature sensitive tape or indicators are included in autoclave loads, and the autoclaves are tested monthly to assure adequate temperature attainment using commercially-available biological indicators (bacterial spore vials) included with the operational load. Protective clothing, such as sterile surgical gloves, face masks, shoe covers and hair covers are discarded after each use. Cloth surgical gowns are laundered and autoclaved before use. Disposable surgical gowns are either purchased sterile, or autoclaved and then discarded after use. Some instruments, such as disposable scalpels, are purchased sterile and disposed of after each use. c. Describe surgical support functions provided by the program to investigators. ULAR Veterinary Services maintains a large animal anesthesia machine with ventilator and monitor and several portable rodent isoflurane anesthesia systems for use by investigators on an as-needed basis. These systems may be used in dedicated surgical suites, animal facility procedure rooms and within investigator labs, if approved as a performance site in an IACUC protocol. ULAR Veterinary Services provides surgical support duties as needed and requested. This includes training and oversight of surgeries, but may also include other surgical, anesthetic, or post-op monitoring duties. 5. Intraoperative Monitoring [Guide, p. 119] Describe monitoring and recording requirements for each species, including the type of record(s) maintained. Also note monitoring of anesthesia during nonsurvival procedures. For rodents, monitoring must include a post-anesthetic pre-surgical assessment to ensure the animal is adequately anesthetized prior to commencement of the surgical procedure. Monitoring during surgery must occur continuously, observing for any signs of movement or increases in respiration or changes in respiratory pattern. Records may be maintained in a laboratory type notebook, and should include date of procedure, animal ID, surgical procedure performed, dosages of anesthetic agents(s) administered. For large animals, additional monitoring may include heart rate, SpO2, end tidal CO2, and blood pressure. Observations must be recorded on an anesthetic record at least every 15 min. 6. Postoperative Care [Guide, pp. 119-120] 71 2/11 Describe the postoperative care program, including who is responsible for overseeing and providing the care, types of records maintained (e.g., perioperative), where the records are maintained, etc. The investigators are primarily responsible for postsurgical care, which must be described in the approved IACUC protocol. Animals must be continuously observed until they have recovered from anesthesia, e.g. can independently maintain themselves in a sternal position. Postsurgical care includes: observing the animal to ensure uneventful recovery from anesthesia and surgery; monitoring vital signs; providing supplemental heating; administering supportive fluids, analgesics, and other drugs as required or approved in the study proposed; and providing adequate care for surgical incisions. Investigators are responsible for maintaining postoperative records. Veterinary Services personnel are available to assist with postsurgical recovery and supportive care, as well for training in postprocedural care. Veterinary Services personnel also provide oversight of postoperative care, reviewing records and providing feedback to investigators and the IACUC if problems arise. E. Pain and Distress [Guide, pp. 120-121] 1. Describe how and by whom pain and distress are assessed and categorized. When submitting an IACUC protocol, the investigator describes the procedures to be performed and defines the criteria to be used to assign the animals to one of the pain categories listed on the annual report to the USDA (APHIS form 7023). An outline of the USDA pain categories is included with the protocol application form. Veterinarians are available to consult with the investigator and can evaluate procedures for pain or distress. The IACUC reviews all procedures, including those with the potential for animal pain or distress, the USDA pain category to which the animals have been assigned and requires protocol adjustments as needed. If the IACUC has particular concerns that a procedure may be high risk for producing post procedural pain or distress, the IACUC will request that a ULAR veterinarian observe such animals when a study begins, initiate appropriate refinements and then report back to the IACUC as part of the IACUC Monitoring and Outreach Program. Animals in the animal facility are assessed for signs of pain or distress by the investigative staff as described in the IACUC protocol, daily by the animal husbandry staff, and when animals are reported for illness or injury by Veterinary Services. 2. Describe how the IACUC/OB ensures that unnecessary pain and distress are avoided (e.g., pilot studies, monitoring by veterinary staff, animal use protocols, humane endpoints, other refinements, etc.). The IACUC requires the appropriate use of anesthetics and analgesics when procedures performed on animals are reasonably considered to produce pain or distress. Such procedures must be identified in the IACUC 72 2/11 protocol and include criteria used to assess pain and distress in the animals. ULAR veterinarians pre-review all IACUC protocols and have the opportunity to discuss with the investigator issues around animal pain and/or distress prior to committee review and approval. Veterinary and IACUC review of protocols includes the assessment of the investigator’s criteria for administration of anesthetics and analgesics, the management plan to assess and treat pain and distress, including unexpected events, the establishment of humane endpoints, the criteria for removal of an animal from the study and euthanasia. IACUC policies and guidelines designed to minimize pain and distress are available to investigators on the ORA website; policies and guidelines have been developed to cover the use of analgesia, humane endpoints, survival surgery, ascites production, use of Freund’s Adjuvant and many other relevant topics. Deviations from IACUC policies and guidelines must be scientifically justified in a protocol before approval by the IACUC. For protocols involving pain and distress, the IACUC may request that a ULAR veterinarian observe animals on a study and report back to the committee as part of the IACUC Monitoring and Outreach Program. Finally, the animal husbandry staff notifies Veterinary Services when an animal is found to be in apparent pain or distress. If the monitoring and management plan described in the protocol has not been followed by the investigator, after any necessary action by a ULAR veterinarian to alleviate pain or distress, these cases are reported immediately to the IACUC Chair and administrator for committee review and possible action. F. Anesthesia and Analgesia [Guide, pp. 121-123] 1. List the agents used for each species. Dosages, routes of administration and drug combination should be included in guidelines and available at the time of the site visit. Describe also any non-pharmacologic means used to diminish pain and distress. SPECIES ANALGESICS USED ANESTHETIC AGENTS USED Alligators Flunixin meglumine Isoflurane, Pentobarbital Axolotls None Tricaine (MS-222) Birds N/A N/A Cats Butorphanol buprenorphine Flunixin meglumine Pentobarbital, ketamine, valium, isoflurane, alpha-chloralose, and acepromazine Fish None Tricaine (MS-222) Frogs Xylazine Benzocaine, Tricaine (MS-222) Snakes Flunixin Isoflurane, Pentobarbital Pigs Buprenorphine, flunixin, meloxicam Ketamine/xylazine, telazol/xylazine, pentobarbital, 73 2/11 isoflurane Rabbits Buprenorphine, butorphenol, EMLA cream Pentobarbital, ketamine, ketamine/xylazine, ketamine/promazine, telazol, isoflurane, lidocaine Rodents Buprenorphine, butorphanol, meperidine, morphine, ketoprofen, carprofen, acetaminophen, flunixin, meloxicam Ketamine, pentobarbital, xylazine/ketamine, isoflurane, avertin, urethane Non-pharmaceutical methods used to diminish pain and distress depends on the procedure that was performed. Some things that may be done include placing food on the bottom of cage post surgery or using food supplements such as commercial breakfast cereals or nutra-gel packs. Some animals may also receive extra enrichment items such as shredded paper, plastic houses or tunnels or blocks of wood for gnawing. 2. Describe how the veterinarian provides guidance and advice to researchers concerning choice and use of anesthetics, analgesics or other pain moderating methods. ULAR veterinarians maintain guidelines for our investigators on anesthetic and analgesic drug choices for our most common species on the ULAR website at http://www.research.uci.edu/ular/index_vet.htm. A ULAR veterinarian pre-reviews each research protocol prior to IACUC review (paying close attention to painful procedures and pain-relieving medication use and requiring modifications as necessary). ULAR Veterinarians are available for consultation regarding painful procedures and on drug selection and proper usage during the protocol submission process and on an as needed basis at any time. Consultation with a ULAR veterinarian is advertised and encouraged via the IACUC protocol application instructions, and our website at http://apps.research.uci.edu/ular/uci/vet_consultation.cfm. Advertising veterinary consultation services as well as discussing general information on the use of anesthetics and analgesics is provided during the required researcher training classes conducted by Veterinary Services. 3. Describe the monitoring of the effectiveness of anesthetics and analgesics, including who does the monitoring. The IACUC protocol requires the investigator to describe their plan for postop monitoring of animals; this includes frequency, who is to perform, what to observe for, and to list analgesics and anesthetics to be used on the protocol, noting the dosage, route of administration, frequency and purpose, and indications when the use is required (i.e.: clinical signs). Using this information during the semiannual inspections, the IACUC targets investigators using anesthetics and analgesics, and examines stored drugs 74 2/11 for expiration date and drug logs for evidence of use. Veterinary staff often interacts with the investigative staff and conducts routine lab visits during rounds or in response to an animal illness or injury report; this allows for additional monitoring of the use of anesthetics and analgesics. As mentioned above, veterinary monitoring of painful procedures may be required as part of the IACUC Monitoring and Outreach program which leads to a more formal review of anesthetic and analgesic use. Large animals recovering from survival surgery are frequently monitored in the recovery room by research staff or Veterinary Services before they are returned to the animal facility. Some animals require the use of the centralized recovery room and/or anesthesia or post-operative care support by Veterinary Services, therefore veterinary staff is sometimes directly involved in the anesthesia and post-operative care process. 4. Describe how the veterinarian(s) and the IACUC/OB evaluate the proposed use of neuromuscular blocking agent to ensure the well-being of the animal. In the IACUC protocol the researcher must identify the paralytic agent(s) and provide a scientific justification for their use. They need to indicate which procedure(s) described in the Experimental Design require the use of paralytic agents and why. A detailed description of how the paralytic agent will be administered must be provided, including the point in the surgical/experimental procedure at which it is first administered and approximately how long animals will be under its influence. They must describe the anesthesia regimen that will be used while the animals are under the influence of the paralytic agent, indicate how the depth of anesthesia will be assessed prior to administration of the paralytic agent and how animals will be monitored for adequate depth of anesthesia while the paralytic agent is in effect. A description must be provided of how mechanical ventilation will be performed while the paralytic agent is in effect, including a description of the equipment used and details of the tidal volume and respiration rate. A surgical plane of anesthesia must be established and verified prior to administration of the paralytic agent; this anesthesia level must be maintained during the entire time that the agent is in effect. Endotracheal intubation and provision for mechanical ventilation must be initiated prior to the administration of the paralytic agent. The use of paralytic/neuromuscular blocking agents should be confined solely to the phase of the procedure for which they are indicated. During the period of paralysis, multiple physiologic indicators of pain and distress (e.g., heart rate, blood pressure) must be monitored at least every 15 minutes as appropriate to the species and recorded in the surgical record. An increase of >20% in any monitored parameter should be considered indicative of a pain/stress response and additional doses of anesthetic must be administered. The use of automated monitoring devices, however, cannot substitute for direct monitoring of the animal by a human observer. A member of the laboratory staff must be present at all times while paralytic agents are in use. The use of end-tidal carbon dioxide monitoring is strongly recommended to ensure adequate ventilation. Core temperature and fluid balance must be maintained within normal levels during the period 75 2/11 of paralysis. In the event that animals will be under the influence of the paralytic for long periods of time (e.g., more than 4-6 hours), a urinary catheter must be placed or the urinary bladder must be manually voided. 5. Describe policies and practices for maintaining and ensuring function of equipment used for anesthesia. IACUC policy requires that all equipment associated with the delivery of inhalant anesthetics be evaluated regularly to assure its proper function and integrity. Anesthetic vaporizers must periodically have the calibrations verified by a professional as recommended by the manufacturer; when manufacturer recommendations are unknown or not available, equipment should be inspected at least once every 3 years and calibrated as necessary. Before each use, the vaporizer and other components should be inspected by the user to ensure that all components are correctly set up. Vaporizers must be tested at least once every three years by an authorized anesthetic machine service provider to verify accuracy of calibration. If the verified anesthetic delivery is > 10% out of calibration, the unit should be serviced by an authorized service provider. Vaporizers should have a certificate of the calibration date affixed directly to the machine/vaporizer after each service. In addition, written manufacturer's recommendations for service of vaporizer systems should be kept in the laboratory and all research staff should be familiar with them. G. Euthanasia [Guide, pp. 123-124] 1. Describe approved methods of euthanasia, including humane slaughter. Include consideration of species, age, condition (e.g., gestational period, or neonatal) and location(s) for the conduct of the procedure. All euthanasia methods must be described in the IACUC protocol and conform to recommendations of the AVMA Guidelines on Euthanasia and the IACUC Policy for Euthanasia of Research Animals at http://www.research.uci.edu/ora/acup/euthanasia.htm. Rodents are primarily euthanatized by CO2 delivered from compressed-gas canisters, followed by a secondary method, such as cervical dislocation, to ensure death. Parenteral (ip) pentobarbital and cervical dislocation or decapitation with prior use of an anesthetic are also commonly used for rodents. Use of cervical dislocation or decapitation without prior anesthesia requires a scientific justification in the approved protocol. Cardiac perfusion is also commonly used. This is also performed in a deeply anesthetized or recently expired animal and the procedure must be describe in the IACUC protocol. Cats, pigs, rabbits, birds, and larger rodents are generally euthanatized using pentobarbital-based products (e.g.: Euthasol®) after initiation of anesthesia. Aquatic animals are overdosed with MS-222. 2. Describe policies and practices for maintaining and ensuring function of equipment used for euthanasia. 76 2/11 The most common piece of equipment used is CO2 delivered from H size compressed-gas canisters. The canister has a regulator and flow valve attached with a tube that goes to either a chamber or can be directly attached to a ventilated mouse cage. This is regularly inspected and maintained by ULAR personnel. Guillotines are also common used, and these are inspected regularly by the users and also by the IACUC during the 6-month facility inspections. The guillotines are mostly used after an animal is anesthetized, or even commonly after death; only rarely are they used on an unanesthetized mouse or rat. 3. Describe the methods used to confirm death of an animal. The IACUC requires a secondary physical method of euthanasia for all rodents after the animal is profoundly anesthetized, prior to carcass disposal. Acceptable secondary physical methods for adult and neonatal rodents include: •Decapitation •Cardiac perfusion •Removal of vital organs (e.g. heart, lungs, brain) •Opening of the chest cavity to induce pneumothorax •Cutting the major blood vessels to induce exsanguination (e.g. aorta, vena cava) •Cervical dislocation may only be used in adult rodents, as it can be difficult to perform in neonates and thus is not appropriate for use in animals prior to weaning. These procedures may not be performed in conscious animals without specific IACUC approval. For confirmation of death in non-rodent mammalian species the following procedures are followed: 1.Heart beat: must be assessed for five minutes or more. The best assessment is through direct palpation of either the pulse in the carotid or femoral artery or direct cardiac palpation. If there is any question, the thorax should be opened, the heart exposed and viewed directly or palpated to confirm lack of activity. Arterial pulse of smaller species may be difficult to palpate, so direct inspection of cardiac mechanical activity is necessary. Lack of electrical activity of the heart as determined by ECG (provided that the leads are correctly connected) may also be utilized to confirm death. 2.Pupillary response to light: Shine a bright light into the eyes of the animal. A constriction (narrowing) of the pupil indicates a neurological response. Upon death, the pupils will become dilated and unresponsive to light. Some drugs and experimental agents (e.g., anticholinergics such as atropine) can prevent pupillary reactivity or otherwise affect this neurological response. 3. Respiratory pattern: Profoundly anesthetized animals may exhibit shallow and irregular breathing patterns that may be confused for lack of spontaneous breathing. Thus, lack of spontaneous breathing should not be used as sole criteria for confirming euthanasia. 77 2/11 For confirmation of death in ectothermic vertebrates a secondary method should always be performed, such as pithing, removal of the heart or decapitation. IV. Physical Plant [Guide, pp. 133-151] Repeat this section for each animal housing area, including agricultural settings, temporary holding areas for field studies, aquatic environments, and each IACUC/OB approved satellite housing facility. Include as an appendix the floor plans of each (if applicable) on 8.5" x 11" or A4 paper. A. Location and Construction Guidelines 1. Note the location (building, floor, wing, etc.) of the animal facility(ies). Describe the management structure and program oversight for each of the areas listed in this section. Biological Science 3: The vivarium is located in the basement of the building. The vivarium is managed by ULAR. McGaugh Hall: The vivarium is located in the basement of the building. The vivarium is managed by ULAR. Bonny Center: The vivarium is located on the first floor in rooms 300A-G. The vivarium is managed by ULAR. Gillespie Neuroscience Research Facility: The vivarium is located in the basement of the building. The vivarium is managed by ULAR. Hewitt Hall- BSL-3: The vivarium is located on the third floor of Hewitt Hall. The vivarium is managed by Gary Landucci. Hewitt Hall: The vivarium is located in the basement. The vivarium is managed by ULAR. Irvine Hall: The vivarium is located on the first floor in room 198. The vivarium is managed by ULAR. Medical Science A: The vivarium is on the first floor. The vivarium is managed by ULAR. Medical Science A Annex: The entire one storey building is the vivarium and it is managed by ULAR. Med Surge II: The vivarium is located in the basement and first floor. The vivarium is managed by ULAR. Natural Science 2 Aquatics Facility: The aquatics facility is located on the third floor. The facility is managed by the Principal Investigators and their staff. North Campus Aviary (including Mate Choice Laboratories): This facility is located on the north campus, approximately 1.4 miles from the School of Biological Sciences animal housing facilities. The vivarium is managed by the Principal Investigators and their staff. North Campus Air Pollution Health Effects Laboratory (APHEL): APHEL is located on the north campus in building 239, rooms 106,107, 107a, 107b, 107c, 107d, 107e, 108a, and 108b. The vivarium is managed by the Principal Investigators and their staff. 78 2/11 Steinhaus Hall: The vivarium is located in the basement. The vivarium is managed by ULAR. UCIMC Building 55: UCIMC Building 55 is located approximately 12 miles from the main campus at the UCI Medical Center, Orange. The vivarium is located in the basement. The vivarium is managed by ULAR. 2. Describe the physical relationship of the animal facilities to the research laboratories where animals may be used. Research laboratories are located on separate floors, in different rooms, or in another building in relation to the vivaria. 3. Describe the general arrangement of the animal facilities (e.g., conventional, clean/dirty corridor, etc.). For animals that are maintained in a laboratory in order to satisfy the scientific aims of a protocol, describe the housing and care provided and the maximum period of stay required. The vivaria are operated as conventional facilities with the exception of: Biological Science 3: 2 suites (4 animal housing rooms) dedicated to the Transgenic Mouse program, which is operated as a Barrier. Hewitt Hall: Entire vivarium is operated as a Barrier. McGaugh Hall: B520 suite (3 housing room) is operataed as a Barrier. Hewitt Hall BSL-3: The vivarium is operated as a Containment/Barrier. In order for researchers to keep animals outside of central vivarium facilities (e.g., in the laboratory) for more than 12 hours, two appendices must be submitted with the protocol for review and approval by the IACUC: Appendix I, which serves as a Standard Operating Procedure for researcher-provided husbandry and care of animals; and Appendix J, the request for exception to standard ULAR-provided vivarium housing. The request for housing exception must include a scientificallybased justification for housing animals outside of ULAR vivarium space. The Principal Investigator and his/her staff are responsible for the husbandry, care and maintenance of the animals at all times while they are outside of the vivarium. 4. Describe finishes throughout the animal facility(ies) for floors, walls, ceilings, doors, alleyways, and gates. Note any areas that are not easily sanitized and describe how these areas are maintained. Biological Science 3: Floors: Resinous floor finish (Neogard-Tan) with integral cove base. Walls: High Impact Wallboard with epoxy coating Ceilings: Gyp Board ceiling with epoxy finish Doors: Hollow Metal doors with epoxy paint. McGaugh Hall: Floors: Resinous flooring (Dur-A-Flex) with integral wall coved base (renovated part); Dexotex TM (other areas) Walls: Gyp Board with epoxy paint (renovated part); Epoxy finish (other areas) 79 2/11 Ceilings: Gyp Board in the corridor with epoxy paint; fully gasketed 2’x4’ extruded aluminum ceiling grid and fiberglass tile (renovated part); Epoxy finish (other areas) Doors: Painted steel doors and polished steel hardware (renovated part); Epoxy finish (other areas) Bonney Center: Floors: Epoxy finish (replaced in 2009) Walls: Epoxy finish Ceilings: Gyp Board ceiling with epoxy finish Doors: Hollow Metal doors with epoxy finish Gillespie Neuroscience Research Facility: Floors: Epoxy Aggregate finish Walls: Glaze-coat fiberglass Ceilings: Glaze-coat fiberglass Doors: Hollow Metal doors with epoxy finish Hewitt Hall- BSL-3: Floors: Sheet vinyl (industrial grade) Walls: Fiberglass reinforced panels over wallboard Ceilings: Fiberglass reinforced panels Doors: Hollow Metal doors with epoxy finish Hewitt Hall: Floors: Resinous flooring (Stoneblend GSI) with integral coved base Walls: Gyp Board with epoxy paint Ceilings: Gyp Board in the corridor with epoxy paint; Fully, gasketed 2’x4’ fiberglass ceiling grid and tile Doors: Stainless steel doors and hardware Irvine Hall: Floors: Dexotex TM covered concrete Walls: Gypsum Board with Epoxy finish Ceilings: Gypsum Board with Epoxy finish Doors: Varnished Wood Medical Science A: Floors: Dexotrex TM troweled-on epoxy. Newly replaced floor (2011-2012) are methyl methacrylate (MMA). The cage wash area has UCRETE floor. Walls: Epoxy paint Ceilings: Main corridor: fiberglass panels with gel coat, Rooms 115, 119, 121, 122A-122H, 112, 114, 116, 118, A124A and A124S, : solid epoxy finished ceilings with recessed lights Doors: Hollow Metal doors with epoxy finish Medical Science A Annex: Floors: Epoxy Walls: Fiberglass reinforced panels over wallboard Ceilings: Fiberglass reinforced panels Doors: Hollow Metal doors with epoxy finish Med Surge II: Floors: Epoxy coated cement Walls: Gypsum Board with epoxy finish Ceilings: Gypsum Board with Epoxy finish Doors: Hollow Metal doors with epoxy finish 80 2/11 Natural Science 2 Aquatics Facility: Floors: Broadcast epoxy coating. Walls: Moisture resistant, impact resistant wall board with epoxy finish Ceilings: Moisture resistant ceiling tiles Doors: Hollow Metal doors with epoxy finish North Campus Aviary (including Mate Choice Laboratories): Floors: Epoxy finish Walls: Epoxy finish Ceilings: Epoxy finish Doors: Epoxy finish North Campus Air Pollution Health Effects Laboratory (APHEL): Floors: Dexotex TM finish Walls: Epoxy finish Ceilings: Epoxy finish Doors: Solid Wood Steinhaus Hall: Floors: Epoxy finish and sheet vinyl Walls: Epoxy finish Ceilings: Epoxy finish Doors: Epoxy finish UCIMC Building 55: Floors: Broadcast epoxy Walls: Epoxy with imbedded fiberglass reinforcement Ceilings: Fiberglass panels with gel coat Doors: Hollow Metal doors with epoxy finish 5. If exterior windows are present within the animal housing or procedure areas, describe IACUC/OB consideration regarding temperature and photoperiod control, as well as potential security risks. With the exception of the Medical Science A vivarium, no exterior windows are present within the animal housing or procedure areas. In Medical Science A, the exterior windows are blacked out with black paint for photoperiod control and security. Room temperature is controlled via our HVAC system. B. Functional Areas and Operations 1. Heating, Ventilation, and Air-Conditioning (HVAC) [Guide, pp. 139-140, 143] a. Describe the mechanical systems used to provide temperature, humidity and air pressure control. Include details such as the use of variable air volume (VAV) systems, and additional key features of HVAC systems affecting performance. Air pressures are controlled by Phoenix Control systems with the exception of Medical Science A Annex, which has a Siemens Control system. Temperatures are controlled by Siemens or Johnsons systems. Humidity is not controlled. 81 2/11 b. Describe construction features that minimize the potential for adverse consequences to animal well-being, such as re-heat coils that fail closed or that are equipped with high-temperature cut-off systems. The majority of the facilities are being monitored through the Building Management Systems, so when they are out of range, an alarm is generated at the Central Plant. The campus standard for all valves is fail closed. That is not to say there are older installations that are otherwise. c. Describe how critical air pressures, ventilation, and temperature are monitored and maintained in the event of a system or component failure. Significant changes (e.g. <64oF or >77oF) in holding room air temperature trigger alarms in the FM control room, which are manned 24-hours per day. In the event of an alarm, FM will dispatch mechanics to troubleshoot the problem. FM will notify ULAR if the problem cannot be resolved in 3 hours or less. Also, temperature and humidity gauges are placed in each animal housing room and checked daily by the animal care staff. In the event of a significant change, UCI Facilities are notified and appropriate actions will be taken. At North Campus APHEL, the HVAC is not on automatic backup – the HVAC for the mouse rooms can be attached to an emergency generator and portable A/C units are brought into the rat rooms for emergency use. If the airconditioner fails at the North Campus Aviary Mate Choice lab, Facilities Management will provide a temporary air-conditioning unit. d. Describe procedures for monitoring animal facility mechanical systems and notifying appropriate personnel in the event of a significant failure that occurs outside regular work hours. Significant changes (e.g. <64oF or >77oF) in holding room air temperature trigger alarms in the Facilities Management control room, which is manned 24hours per day. In the event of an alarm, FM will dispatch mechanics to troubleshoot the problem. FM will notify ULAR if the problem cannot be resolved in 3 hours or less and the appropriate action is initiated to respond to the emergency. 2. Power and Lighting [Guide, p. 141] a. Note if emergency power is provided for the animal facility and if so, what electrical services and equipment are maintained in the event the primary power source fails. With the exceptions of North Campus facilities, the vivarium corridor lights and emergency exit signs are maintained by emergency power. Biological Science 3: All animal holding rooms have emergency power outlets. IVC animal racks’ air supply and/or exhaust blowers are plugged into the emergency power outlets. 82 2/11 McGaugh Hall: About 80% of IVC air supply and/or exhaust blowers are plugged into emergency power outlets. The remaining 20% consist of static housed animals and housing rooms without emergency power. Bonney Center: The animal housing rooms do not have emergency power. Gillespie Neuroscience Research Facility: Most animal housing rooms do not have emergency power with the exception of the follow rooms: B101 (housing room): The biological safety cabinet is plugged into e-power B241 (housing room): Has one outlet on e-power; however no equipment is plugged into it. Hewitt Hall- BSL-3: All animal support systems are on emergency power (includes HVAC system). Hewitt Hall: IVC animal racks and surgical room equipment is plugged into the emergency power outlets. Irvine Hall: The housing room does not have emergency power. Medical Science A: The housing rooms do not have emergency power. Medical Science A Annex: The housing rooms do not have emergency power. Med Surge II: Emergency power outlets are available in holding rooms and procedure room. In B341, an animal change station is plugged into an emergency power outlet. Other emergency power outlets are used for a dedicated piece of equipment. Natural Science 2 Aquatics Facility: Aquatic animal housing systems in Nat Sci II are on emergency power. North Campus Aviary (including Mate Choice Laboratories): The facility is not on emergency power. North Campus Air Pollution Health Effects Laboratory (APHEL): In the event of a power failure, Facilities Management will provide a backup generator. Steinhaus Hall: The animal housing rooms do not have emergency power. UCIMC Building 55: Room 048 (swine housing) has emergency power outlets; however, no equipment is plugged into it. Other animal housing rooms do not have emergency power. The vivarium surgery rooms have emergency power outlets and surgical lights, monitoring devices, tools…etc are plugged into the e-power outlets. b. Give history of power failures for the animal facility. Note frequency and duration. If emergency power was not available during a power failure, describe steps taken to ensure the comfort and well-being of the animals and the temperature extremes reached in the animal rooms during the failure. We experienced four outages in the last 3 years. However, it took less a minute for the backup generator to kick into operation. In Nov. 2009 the chilled water supply for Gillespie and Hewitt Hall was interrupted for a 4 hour period. This chilled water supplied cool water to power the air conditioning. During this period animal room temperatures reached 80ºF. 83 2/11 Temperatures in the animal rooms returned to normal as soon as the line was repaired and no animal deaths or health issues resulted. c. Describe lighting system(s) for the animal housing facility(ies). For each species or holding room type, list light intensity, photoperiod (Light:Dark), construction features (e.g., water resistance), and control (e.g., automatic versus manual, phasing). For systems automatically controlling photoperiod, describe override mechanisms. Species Light Intensity All 300-480 lux (1 m off floor) Photoperiod (L:D) 12:12 – Majority of rooms 14:10 – Some mouse breeding rooms Water-resistant light fixtures (yes/no) Yes Automatic control (yes/no) Yes Windows (yes/no) No Lights cycles can be overridden via an override switch at each animal housing room. 3. System Malfunctions. If not previously reported, describe animal losses or health problems resulting from power, HVAC, or other life support system (e.g., individually ventilated cages) failures, and mechanisms for reporting such incidences. AAALAC International Rules of Accreditation (Section 2.f) We have had no animal losses or animal health problems resulting from system failures. 4. Storage Areas [Guide, pp. 141-142] a. Describe storage areas for feed and bedding, including temperature and vermin control. Feed is delivered weekly. The mill dates are checked upon arrival and stored to ensure first-in first-out in feed rooms within the animal facility or a central storage building. Temperature in all of the storage facilities is maintained at 68°-72°F and floors are swept and mopped weekly. Vermin control is accomplished through exclusion barriers, immediate sanitation of any spilled product, daily observation for any sign of infestation, and use of baited spring traps. On the north campus, bird seed is stored unopened at room temperature until 3 days prior to use, at which time it is placed into a freezer for 3 days. The freezing procedure kills immature forms of moths and beetles that are frequently found in these seed mixtures, but does not compromise the nutritional value of the seed and the seed still germinates after treatment. The seed is then transferred to lined, plastic garbage cans and used immediately. These garbage cans are labeled and have tight-fitting lids. Most food 84 2/11 supplements are kept refrigerated, but grit and cuttlebone are maintained at room temperature in plastic garbage cans. b. Describe storage areas for cages, equipment, supplies, etc. Cages and supplies are stored in designated rooms within the vivaria. c. Describe storage areas for flammable or hazardous agents and materials (e.g., disinfectants, pesticides, fuel). Flammable or hazardous agents are stored in cabinets or secondary containers appropriate for the specified hazards. 5. Facilities for Sanitizing Materials [Guide, pp. 153] Describe for each cage sanitation area its location, the traffic flow pattern (soiled to clean, or in and out) within the facility, and kinds of equipment (tunnel washer, bottle washer, rack washer, etc. and other related equipment such as bedding dispensing units). Biological Science 3 1) Location: The sanitizing facility is located in B640/dirty side, B620/clean side and B610/sterile cage room. 2) General Features: The traffic flow pattern is from the dirty to the clean side of the cage wash area. Soiled bedding is removed from the cages on the dirty side and the cages are loaded into the cage washer. After sanitization, the clean cages are removed on the clean side for storage or use. Clean items are autoclaved and wrapped barrier operations. 3) Equipment: Within these areas are one pass-through tunnel washer with garbel/vacuum unit and bedding dispenser, a double door Lynx rack washer, a small autoclave and a large, pass-through sterilizer to support barrier operations locally as well as in Hewitt Hall and the Annex facilities. McGaugh Hall: 1) Location: The sanitizing facility is located in B502/dirty side, B500/clean side and B500A/sterile cage room. A pass-through sterilizer is also located within the B451 suite to process biohazard items out of B451 suite. 2) General Features: The traffic flow pattern is from the dirty to the clean side of the cage wash area. Soiled bedding is removed from the cages on the dirty side and the cages are loaded into the cage washer. After sanitization, the clean cages are removed on the clean side for storage or use. Clean items are autoclaved and wrapped for barrier operations. 3) Equipment: The facility has one pass-through tunnel washer (including garbel and bedding dispenser) and rack washer. On the dirty side is a front-loaded sterilizer to process dirty items prior to washing. The clean side cage washer has a pass-through sterilizer which is used to support barrier housing supply needs. A pass-through sterilizer is also located within B451 suite to process biohazard items out of the suite. 85 2/11 Bonney Center: 1) Location: The Bonney Center has a double door Better Built rack washer in a “dirty” and “clean” space (room 306) at the end of the vivarium (there is no physical separation between the two sides). 2) General Features: Soiled cages are entered through the “dirty” side, bedding removed, cages washed, unloaded from the “clean side,” then rolled down the corridor to the clean cage area. The floor and walls are sealed concrete and the ceiling is sheet rock construction with epoxy finish. 3) Equipment: Double-door Better Built rack washer. The washer was installed without steam service; it uses 100 ppm sodium hypochlorite to sanitize caging equipment Gillespie Neuroscience Research Facility: 1) Location The dirty side of the cage wash is located in room B243; the clean side is located in room B209. 2) General Features: The traffic flow pattern is from the dirty to the clean side of the cage wash area. Soiled bedding is removed from the cages on the dirty side and the cages are loaded into the cage washer. After sanitization, the clean cages are removed on the clean side for storage or use. 3) Equipment: A Basil 4600 pass-through cage and rack washer and a Lynx tunnel washer is installed. It has recovery tanks for optional reuse of acid and alkaline detergent solutions as well as cool-down of waste discharge. Hewitt Hall- BSL-3: The BSL-3 suite includes a pass-through autoclave for sterilization of material exiting the suite. Once waste has passed through the autoclave it is treated as nonhazardous soiled material and processed as routine waste in the Gillespie vivarium. The Gillespie vivarium cage wash includes a double door, pass-through rack washer and tunnel washer. Hewitt Hall: Soiled materials are transported to Biological Science 3 cage wash area for sanitization and cleaned materials are transport back to Hewitt for use. Irvine Hall: Soiled materials are transported to Gillespie Neuroscience Facility vivarium cage wash area for sanitization and cleaned material are transport back to Irvine Hall for use. Medical Science A: 1) Location: The Med. Sci. A cage cleaning facility is in Room 108 (dirty side) and Room 102 (clean side). 2) General Features: The traffic flow pattern is always from the dirty room to the clean room. Soiled cages enter the dirty side, bedding dumped, cages washed, unloaded and dried in the clean side. The bedding is then added and the cages are rolled down the corridor on carts to the animal housing area. 3) Equipment: Med. Sci. A has a double door, IWT rack washer, a pass-through Scientek bottle washer, a double door Eagle 3000 pre-vacuum sterilizer, size 24" x 36" x 5', located in room 102 (clean side cage wash area) and an acid dip tank used to remove urine scale from rabbit pans. 86 2/11 Medical Science A Annex: Soiled materials are transported to Gillespie Neuroscience Facility/Biological Science 3 vivarium cage wash area for sanitization and cleaned material are transport back to Med Sci A Annex for use. Med Surge II: Soiled materials are transported to Medical Science A Facility vivarium cage wash area for sanitization and cleaned material are transport back to Med Surge II for use. Natural Science 2 Aquatics Facility: Tanks are washed within housing rooms (in large tub sinks) or in the shared support area. Washing is performed manually be laboratory personnel. North Campus Aviary (including Mate Choice Laboratories): 1) Location: Room 101 in the Wild Life Vivarium, room 3 in the Mate Choice Laboratory. 2) General Features: The cages are brought to the sanitizing areas where they are hand cleaned and taken back to the animal rooms. 3) Equipment: A dishwasher is located in room 101, but most of the equipment is washed by hand. North Campus Air Pollution Health Effects Laboratory (APHEL): 1) Location: Cage sanitation facility is located behind Bldg. 238. Cages are rolled from rooms 108a and/or room 107 to the cage washer. 2) General Features: The APHEL facility is too small to have separate traffic lanes for clean and soiled. The animal care personnel are required to change gloves and lab coats depending on the function that they are performing. 3) Equipment: North campus has its own cage and rack washer to sanitize the rodent cages. The cage washer is located outside, enclosed by three walls and a roof. The rack washer and steam generator are located next to the cage washer but are fully enclosed. Steinhaus Hall: Most animal cages used in Steinhaus Hall are disposable; some soiled materials are transported to McGaugh Hall vivarium cage wash area for sanitization and cleaned material are transport back to Steinhaus Hall for use. UCIMC Building 55: 1) Location: The clean side of the cage wash is room 084A and dirty side is room 084B. 2) General Features: The traffic flow pattern is from the dirty to the clean side of the cage wash area. Soiled bedding is removed from the cages on the dirty side and the cages are loaded into the cage washer. After sanitization, the clean cages are removed on the clean side for storage or use. Clean items are autoclaved and wrapped barrier operations. 3) Equipment: Cages are sanitized using a rack washer. A ventilated bedding dump station is available in the soiled cage wash. A pass through autoclave is also available. The clean cage wash includes an automatic bedding dispenser. C. Special Facilities [Guide, pp. 144-146, 150] 87 2/11 1. Specialized Types of Animal Housing Note specialized types of available animal housing spaces such as barrier, hazard containment (infectious, radioactive, chemical), "animal cubicles" (also known as "Illinois Cubicles”, "Horsfal Cubicles," and "animal modules"), or facilities designed specifically for housing certain species such as aquatic or agricultural animals (e.g., barns, feedlots). [Guide, pp. 160-161] Barriers: Hewitt Hall was designed to operate as a rodent barrier facility. Biological Sciences 3 has 2 suites of rooms and McGaugh Hall has one suite of rooms operated as a barrier facility. Hazard containment: McGaugh Hall has an ABSL2 containment suite (B451), consisting of two holding rooms, one procedure room and a cage processing room with a pass-through autoclave. Hewitt Hall has an ABSL3 facility on the 3rd floor that was designed and commissioned for use with ABSL3 agents, including Select Agents. It consists of two holding rooms, Cubicles: Med Sci A has two rooms with cubicles. One of these rooms (124S) is used for small ABSL2 studies. The other room (124A) is used to house small numbers of animals that need to be housed separately from other animals for experimental reasons or because they are a different species. These cubicles are only used for rodent studies. Gillespie has one room with cubicles (223). This room is used to house small numbers of animals that need to be housed separately from other animals for experimental reasons or because they are a different species. These cubicles are only used for rodent studies. Nat Sci 2 Aquatic Housing: Nat Sci 2 has a dedicated aquatic vivarium. Species currently housed here include zebrafish, Xenopus (laevis and tropicalis), axolotls, tilapia, and goldfish. North Campus Aviary: North Campus Aviary has outdoor flight cages specifically designed to house small birds, such as finches and budgerigars. 2. Surgery [Guide, pp. 144-145] a. Describe facilities for aseptic surgery, surgical support, animal preparation, surgeon’s scrub, operating room, and postoperative recovery. Rodent and minor and non-survival large animal surgeries are performed in a number of locations (see list in Section III.D.2.a.) These locations vary from dedicated procedure rooms within the animal facility to bench space within a research laboratory. Major large animal survival surgery is performed in one of three dedicated surgical facilities. These are located in Beckman Laser Institute, Medical Science A, and UCI Medical Center building 55. Each of these locations has a surgical room that is separate from the animal prep room and surgeon’s scrub room. 88 2/11 b. Describe construction features of the operating room(s), including interior surfaces, ventilation, lighting, and fixed equipment used to support surgical procedures and enhance contamination control. Construction features of rodent and minor or non-survival large animal surgical facilities vary by location. In some locations the facility may consist of a dedicated bench space within a research lab. In others the facility consists of a dedicated procedure room within the animal facility that includes features such as positive air flow, impervious floors, walls and ceilings, stainless steel or surgical tables, fixed surgical lights, emergency powered electrical outlets, piped-in oxygen supply and built-in gas scavenging system. The 3 dedicated large animal survival surgical facilities have epoxy coated floors and walls with moisture resistant fiberglass ceiling panels or epoxy coated plaster. The main surgical room is positively ventilated with coarse filtered air. The surgical room is equipped with fixed surgical lights, piped in oxygen or Htanks secured to the wall and built-in gas scavenging system. The surgical support area in each facility contains a built-in autoclave. 3. Other Specialized Animal Use Facilities [Guide, pp. 146-150] Describe other facilities such as imaging, irradiation, and core behavioral laboratories or rooms. Include a description of decontamination and methods for preventing cross-contamination in multi-species facilities. Specialized imaging (MRI, PET, Fluorescent, two-photon microscopy, optical coherence tomography (OCT)) facilities are located in Irvine Hall, Med Sci C, Med Sci Annex, Beckman Laser Institute, and McGaugh Hall. A cesium based irradiator is located in Med Sci B. These facilities are located in dedicated rooms within the animal facility or in research lab areas. Facilities and equipment are cleaned and decontaminated after each use, with special emphasis placed on disinfecting any animal contact areas. Most of the animal work performed is with SPF status rodents. Several human clinical facilities located at UCIMC are occasionally used for animal studies (see Section I.A.2.b.ii.4) for a description, including procedures followed for prevention of cross-contamination.) 4. Other Animal Support Facilities Describe other facilities providing animal care and use support, such as food preparation areas, feedmills, abattoirs, etc. N/A D. Security and Access Control [Guide, p. 151] Describe such features as control of entry, perimeter fences, gates, entryways, cameras, guards. A photo card-key is required to gain entrance to the most vivarium areas, with the exception of Nat Sci 2 Aquatic Facility, North Campus APHEL and North Campus Aviary and Mate Choice Labs. The photo card keys must be worn 89 2/11 visibly at all times while in the vivaria. The card-key security system is used to enter the building after hours and on holidays and weekends. The system is alarmed directly to the UCI Police Department. All laboratory and vivarium facilities are patrolled on the exterior by UCI police. Video security cameras are positioned at all entrances and exits for the following vivaria: Biological Science 3, McGaugh Hall, Med Sci A, Med Sci A Annex, GNRF, Hewitt Hall, Steinhaus Hall, Bonney Center. The video feeds are record on DVRs and can be played back in the event of a security breach. Hewitt ABSL 3 Facility is secured with electronic locks that use both biometric and card key access control. Video security cameras are positioned inside and outside the facility. Images are stored on a DVR in the facility manager’s office, and the images are accessible by UCI Police Department, 90 2/11 91 2/11 November 21. 2012 James W. hicks. Ph.D. :\sSociate Vice Chancellor tar Research Office of Research University of California-Irvine 5171 California Avenue. Suite 150 Irvine, CA 92697-7600 Dear Dr. Hicks: The Council on Accreditation of the Association for Assessment and Accreditation of Laboratory Animal Care (AAALAC International) has reviewed the report of the recent site visit to the University of California, Irvine. California. The Council commends you and the staff for providing and maintaining a high quality program of laboratory animal care and use. Especially noteworthy were the strong administrative support for the animal care and use program; the clean and well maintained animal facilities; the knowledgeable and enthusiastic facility managers; the overall good health of the animals; the well written Program Description: the effective oversight by environmental health and safety personnel. demonstrated by the improvement in the review of protocols involving hazardous agents, interactions with the principal investigators, and the provision of training for those working with hazards; the excellent documentation of postoperative monitoring on spinal cord injury protocols, including all supportive care and medications administered: and the well managed surgical center, evidenced by the detailed monitoring records. the well organized facility, and the extremely knowledgeable manager. The Council is pleased to inform you that the program conforms with AAALAC International standards as set forth by the Guide for the (‘are and Uve of tahoraiorv Animals. NRC 2011. Therefore. FULL ACCREDITATION shall continue. Council acknowledges receipt of the correspondence dated September 13. September 6. August 16. and July 9, 2012 detailing actions taken relative to concerns expressed h’ the site visitors during the exit briefing. Specifically, the items addressed satisfactorily included: ensuring the oversight of protocols 19982023 and 1998-201 9 so that appropriate anesthesia and analgesia are provided: reassessing the ease of egress and dc-energizing mechanism of the rack washer in the Bonnev Center: classifying deficiencies noted in the semiannual inspections as significant or minor, developing a plan for their correction, and modifying the semiannual inspection template; providing justification for the single housing of social species of animals: revising Protocol 2001 -2303 to include information regarding experimental side effects; ensuring that documentation of husbandry practices provided by an investigator conforms with Institutional Animal Care and Use Committee policy: verifying that servicing of anesthesia vaporizers is performed in accordance with the manufacturers recommendations: re-evaluating the anesthetic waste scavenging device in BioScience 3. Room B2 1 5 to ensure the protection of personnel: assessing the effectiveness of sanitation procedures of hand—washed primary enclosures; and erifying the relative air pressure differential of the surgery room in the Gillespie Neuroscience Research Facility. ‘ James \V. Hicks. Ph.D. November 21. 2012 Page 2 Council has no further recommendations to oiler tbr improvement of the animal care and use program at this time. We look forward to tbilo ing your program activities and wish on continued success. Ar\A[:\C International requires an Annual Report detailing changes made during the year in accredited units. In the interim, AAALAC International expects to he apprised in a timely manner of significant programmatic changes or concerns should they occur. Please note that, at your request, AAALAC International . ill provide your institution with a separate letter simply verifying that our animal care and use program is accredited. Should you also wish to distribute an electronic copy of this letter to program staff a Portable Document Format (pdf) version will he sent upon request. Sincerely, William W. King. D.V.M.. Ph.D. President, Council on Accreditation WWK:cmw 000238 cc: Jeffrey L. Goodwin, D.V.M.. Ph.D.. Director. University of Laboratory Animal Resources