Isolation of an Aerobic Denitrifying Bacterial Strain from a Biofilter for
advertisement

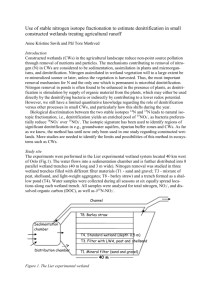
Aerosol and Air Quality Research, 13: 1126–1132, 2013 Copyright © Taiwan Association for Aerosol Research ISSN: 1680-8584 print / 2071-1409 online doi: 10.4209/aaqr.2012.07.0199 Isolation of an Aerobic Denitrifying Bacterial Strain from a Biofilter for Removal of Nitrogen Oxide Zhidao Wu1,2, Shaobin Huang1,2,3,4*, Yunlong Yang1,2, Fuqian Xu1,2, Yongqing Zhang1,3*, Ran Jiang3,5 1 College of Environmental Science and Engineering, South China University of Technology, Guangzhou, China Guangdong Provincial Key Laboratory of Atmospheric Environment and Pollution Control, South China University of Technology, Guangzhou, China 3 The Key Laboratory of Environmental Protection and Eco-Remediation of Guangdong Regular Higher Education Institutions, Guangzhou, China 4 State Key Laboratory of Pulp and Paper Engineering, South China University of Technology, Guangzhou, China 5 Pearl River Water Resources Institute, Pearl River Water Resources Commission, Guangzhou, China 2 ABSTRACT A strain of bacteria CP1 with high nitrogen removal efficiency was newly isolated from the biofilm of a biofilter for removal of NOx from flue gas. The isolate was identified as Pseudomonas aeruginosa based on its physiological and biochemical characteristics and the results of 16S rRNA gene homology analysis. The new isolate had a high denitrifying ability, removing 98.49% of the nitrate in a 24-h period under aerobic conditions, with no nitrite accumulation. With regard to the nitrogen balance, the percentage of nitrogen lost in the flask culture was estimated to be 32.3%, which was presumed to be converted to nitrogen gas. An analysis of its denitrification activity showed that the optimal C/N and temperature were 12 and 30°C–40°C, respectively. By using glucose, sodium citrate and succinate, CP1 removed nitrate with high denitrification efficiency. The change in DO did not influence the effects of denitrification when it varied from 0 to 7.2 mg/L. The results show that CP1 could be a good candidate for the process of aerobic denitrification. Keywords: Aerobic denitrification; Pseudomonas aeruginosa; Nitrogen removal; Isolation and identification. INTRODUCTION Nitrogen oxides (NOx) generally comprises of N2O, NO, NO2, N2O5, N2O3, and NO5. However, this term often refers specifically to nitric oxide (NO) and nitrogen dioxide (NO2), both of which are hazardous air pollutants that lead to the formation of acid rains, airborne particulates, and increase ground level ozone (Barman and Philip, 2006; Han et al., 2011). Emissions of NOx in China have undergone severe increase over the past decade, thus an effective technology is needed to decrease its concentration in the air (Chang et al., 2012). However, the traditional methods used to control NOx emissions from power plant, such as selective catalytic and non-catalytic reduction, require high temperatures or the use of catalysts, resulting in high installation and maintenance costs, and possibly cause secondary pollution (Flanagan et al., 2002; Jin et al., 2005). * Corresponding author. Tel.: 86-20-39380587 E-mail address: chshuang@scut.edu.cn; zhangyq@scut.edu.cn In comparison, the biological method by which nitrogen oxides are converted to nitrogen gas is a promising alternative (Hsieh et al., 2011). Among these methods, a biotrickling filter (BF), usually comprising inert packing material which can provide sufficient surface area for gas/liquid mass transfer and biofilm growth, is a good example and has been applied to the flue gas treatment (Jiang et al., 2009a, b). Denitrification was considered to be an anaerobic process by previous investigators as the enzyme system was inhibited under certain oxygen concentrations (Apel and Turick, 1993; Ferguson, 1994). However, with the increasing number of reports on denitrifying bacteria, aerobic denitrification attracted a lot of attention for its easier operation and higher denitrification rate than anaerobic denitrification. There are recent reports of aerobic denitrifying species isolated from canals, ponds, soils, and activated sludge that can simultaneously utilize oxygen and nitrate as electron acceptors. These include Paracoccus (Lukow and Diekmann, 1997), Pseudomonas (Kesser et al., 2003), Bacillus (Kim et al., 2005), Alcaligenes (Robertson and Kuenen, 1983), and etc. We had developed a BF system where a mature biofilm grew in the filter for 18 months with the property to Wu et al., Aerosol and Air Quality Research, 13: 1126–1132, 2013 remove NOx from desulfurized flue gas at the temperature of about 40°C to 50°C in a coal-fired power plant. The flue gas contains 5–10 percent of O2. NOx removal efficiency reached more than 85% in the best condition when the flow of the flue gas was approximately 10000 m3/h. In biotrickling filter system, NOx in the vapor phase are absorbed by the biofilm and part of NOx can be oxidized into nitrate by both nitrification and chemical oxidation under aerobic conditions (Wang et al., 2006; Reddy et al., 2012). Thus, toxic nitrogen oxides, nitrate and nitrite are reduced to inert nitrogen gas by denitrifying bacteria in liquid phase. To achieve treatment system that has a consistently high efficiency, we should first understand the characteristics of microorganisms resided in the BF system. Therefore, we isolated one of the dominant aerobic denitrifier from the BF system and investigated its denitrification activity under different environment factors to find an effective kind of aerobic denitrification bacteria and to provide useful information for operating aerobic denitrification process in the biofilter system engineering application. MATERIALS AND METHODS Media To screen the aerobic denitrifiers, we used the modified seed medium (SM) and bromothylmol blue (BTB) medium. The SM consisted of (g/L): peptone 8.6, NaCl 6.4, KNO3 1.0, sodium succinate 1.5 at pH 7.0. The formula of BTB medium included (g/L): KNO3 5.0, Na2HPO4·7H2O 7.9, KH2PO4 1.5, MgSO4·7H2O 0.1, disodium succinate 15.0, agar 10.0, BTB (1% in ethanol) 1 mL/L, trace element solution 2 mL/L at pH 7.0. To test denitrification activity, we used a modified denitrification medium (DM) containing the following components (g/L): KNO3 1.0, Na2HPO4·7H2O 7.9, KH2PO4 1.5, MgSO4·7H2O 0.1, disodium succinate 9.4, trace element solution 2 mL/L at pH 7.0. The trace element solutions for the bacterial growth consisted of the following components (g/L): EDTA 50.0, ZnSO4 2.2, CaCl2 5.5, MnCl2·4H2O 5.06, FeSO4·7H2O 5.0, (NH4)6Mo7O2·4H2O 1.1, CuSO4·5H2O 1.57, CoCl2·6H2O 1.61 at pH 7.0. Screening of Aerobic Denitrifier Aerobic denitrifying organisms were enriched from the biofilm of an on-site BF for removal of NOx from the flue gas at the Ruiming coal-fired power plant (Guangzhou, China). The biofilm samples were put into 100 mL of the SM in 250-mL flasks with eight layers of gauze and incubated at 30°C on a rotary shaker at 160 rpm for 1d. A 10% (volume ratio) of bacteria suspension was added to freshly autoclaved SM and incubated for 1d under the same conditions. This procedure was repeated three times. After doubling dilution, the resulting bacterial suspension were streaked onto BTB medium agar plates and incubated at 30°C for 1–3 days. This method was based on the increase in the pH of the medium due to consumption of NO3– by denitrification (Takaya et al., 2003). Blue colonies were selected from the BTB agar plates, transferred to DM and incubated on a rotary shaker. The bacteria suspension with high nitrogen removal rate was streaked onto BTB 1127 again. The rescreening was repeated three times. One of the main strain was isolated from the biofilm, which was named CP1. Identification of the Isolated Bacterium Standard physiological and biochemical characteristics were examined according to previously described methods (MacFaddin, 1984). For pure cultures, 1 mL of log phase cultures was centrifuged for 1 min at 12,000 rpm. The pallet was used for DNA extractions with a bacterial genomic DNA isolation kit (Bioteke Corporation, Beijing, China). The genes encoding 16S rRNA were amplified by using the total DNA (0.1 μg) as the template. PCR was performed in a Mastercycler gradient (Eppendorf 5331, Germany) using the following primers: 16SF (5′-AGAGTTTGATCATGGC TCAG-3′) and 16SR (5′-GGTACCTTGTTACGACTT-3′). Genes were amplified by 30 cycles of denaturation at 94°C for 1 min, annealing at 56°C for 1 min, and extension at 72°C for 2 min, followed by final extension at 72°C for 7min. The PCR products were sequenced by Invitrogen Company (Shanghai, China). The 16S rRNA sequences were examined in blastn (NCBI, USA) for similarities. Aerobic Denitrification by the Isolated Bacterium Capacity of Isolate for Aerobic Denitrification After precultured in SM at 30°C on a rotary shaker at 160 rpm for 12 h, the isolated bacterium (1% v/v) was inoculated to 100 mL of DM and incubated under the same condition for 24 h. Samples were taken from the flasks every three hours for measurement of optical density (OD), nitrate, nitrite, pH and total nitrogen (TN). All tests were performed in triplicate. Effect of Various Conditions The same amount of isolates was cultivated in flasks, and 1% of those mixed cells were used as an inoculum. The inoculum was inoculated to modified DM (varying C/N ratios, carbon sources and dissolved oxygen (DO)) and incubated under different temperature for 24 h. Disodium succinate and potassium nitrate were used as the carbon and nitrogen sources, respectively. The C/N ratios in the medium were adjusted to 1, 3, 6, 9, 12 and 15 by changing the amount of the carbon source and by maintaining a constant nitrogen concentration at 138.5 mg/L. To investigate the electron donor specificity of CP1, glucose, citrate, sucrose, acetate, and succinate were added to the DM as carbon sources, respectively. The concentration of DO and temperature were adjusted by the rotary shaker. After 24 h of cultivation under different environment factors, the concentrations of nitrate, nitrite and OD in the DM were analyzed. All of the experiments were performed in triplicate. Analytical Methods The optical density was determined by spectrophotometry (TU1810; Pgeneral, China) at a wavelength of 600 nm. The estimations concentration of NO3–-N and TN were determined based on standard procedures as described in Standard Methods (APHA, 1992). Meanwhile, NO2–-N was determined by colorimetry at a wavelength of 540 nm Wu et al., Aerosol and Air Quality Research, 13: 1126–1132, 2013 1128 (Murray, 1994). DO and pH were measured with a multifunctional water quality monitor (Mnlti 340i; Germany WTW). Definitions and Efficiency Reporting The efficiency of denitrification was expressed as denitrification efficiency (DE) and accumulation rate of nitrite (NA), defined in Eqs. (1) and (2), as a function of the initial inorganic-nitrogen (Ci0) and last inorganic-nitrogen (Ci1), the initial nitrite-nitrogen (Cn0) and last nitrite-nitrogen (Cn1). C DE 1 i1 100% Ci 0 (1) C Cn 0 NA n1 Ci 0 (2) 100% RESULTS AND DISCUSSION Isolation and Identification of the Aerobic Denitrifying Bacterium CP1 Eight strains were isolated from the first screening and they formed blue colonies and/or halos on the BTB agar plates due to an increase of pH. After rescreening, the nitrateremoval rate was examined in DM under aerobic conditions. One strain, which we designated CP1, had a 97.5% nitrate removal rate and was selected for further characterization. It stained gram negative and showed catalase and oxidase activities (Table 1). Strain CP1 was grown on succinate agar plates in opaque, round, smooth, sandy beige, wet-looking colonies. A BLAST search of available data in the DDBJ/ EMBL/GenBank database showed a high similarity (99.0%) with Pseudomonas aeruginosa strain N002 (Fig. 1). Thus, we designated this bacterium Pseudomonas aeruginosa strain CP1. Aerobic Denitrification by the Isolated Bacterium Capacity of Isolate for Aerobic Denitrification The time for nitrate, nitrite, TN reduction and the growth of strain CP1 are shown in Figs. 2(a) and 2(b).The first six hours were the lag phase. When cell growth reached the exponential phase, the cell concentration increased quickly from 0.099 to 1.133 OD600 (optical density at 600 nm), and the nitrate and TN concentration decreased rapidly. There was no significant change during the stationary phase. After 24h, the nitrate had decreased to 2.06 mg/L from the initial 136.87 mg/L with no nitrite accumulation (dentrification efficiency 98.49%). But the concentration of nitrite increased at first and then decreased during the whole denitrification process. Nitrite peaked at 87.8 mg/L after 12 h of cultivation and was exhausted at 18 h, probably because the activity of nitrite reductase did not enhance until the concentration of nitrite accumulated to a certain amount. As shown in Fig. 2(b), the pH increased from 7.13 to 9.23 and TN decreased from 137.76 to 93.25 mg/L after 24 h of cultivation. From the nitrogen balance, the percentage of nitrogen lost in the flask culture was estimated to be 32.3%, which was presumed Table 1. Taxonomical characteristics of the thermophilic aerobic denitrifying CP1. Test Result Morphological test Colony morphology Round Margin Irregular Elevation Applanation Surface Smooth Density Opaque Pigment Sandy beige Gram’s reaction – Shape Rod Size Short Physiological test Growth temperature 4°C – Growth temperature 41°C + Growth under anaerobic condition + Biochemical test Oxidation/fermentation (O/F) O Catalase test + Oxidase test + H2S production – Voges Proskauer test – Gelatin liquefaction + Nitrate reduction + D-Glucose fermentation + Fructose fermentation + Lysine decarboxylase – ο-Nitrophenyl-β-D-galactopyranoside – Amylolysis test – Pyocyanin test + Methyl red test – Arginine dihydrolase + Citrate utilization + (+) positive reaction; (–) negative reaction. to be converted to nitrogen gas. These results demonstrate that CP1 is an aerobic denitrifying bacterium. Effect of C/N Ratios on Cell Growth and Denitrification The precultured isolated bacterium (1% v/v) was inoculated into the DM with different C/N ratios and incubated at 30°C for 24 h under aerobic conditions. The effect of C/N on the denitrification efficiency is shown in Fig. 3. At a C/N ratio of 1, the value of OD600 was extremely low and the denitrification efficiency was only 5.68%. Removal efficiency and cell growth both increased as more carbon was added to the medium. While the C/N ratio was increased to 12, the denitrification efficiency reached to 97.91% and there was no significant change even if the C/N ratio continued to grow. However, the value of OD600 was still on the increase while the C/N ratio increased from 12 to 15, probably as a result of the fact that the electron flow provided energy for cell growth by utilizing oxygen as electron acceptors while the nitrogen sources were exhausted. When the C/N ratio was less than 12, the strain growed at an insufficient carbon concentration, and therefore the electron flow was too low to provide enough energy for Wu et al., Aerosol and Air Quality Research, 13: 1126–1132, 2013 cell growth. Because carbon was essential for cell growth and nitrate reduction processes, the optimal quantity of carbon was a key parameter in the denitrification process 1129 (Patureau et al., 2000). Therefore, it was important to optimize the C/N ratio for each denitrifier. In this study, the optimal C/N ratio of CP1 was 12. c/mg·L-1 (a) 140 1.4 120 1.2 100 1 80 0.8 nitrate nitrogen nitrite nitrogen OD 60 40 0.6 0.4 20 0.2 0 0 0 3 6 9 12 t/h 15 18 21 24 140 9.5 130 9 120 8.5 pH c/mg·L -1 (b) OD600 Fig. 1. Phylogenetic tree based on a comparison of the 16 S rRNA gene sequence. The phylogenetic tree was generated using the neighbor-joining method. Bootstrap values, expressed as percentages of 1000 replications, are given at branching points. Bar shows two nucleotide substitutions per 1000 nucleotides. TN 110 8 pH 100 7.5 90 7 0 3 6 9 12 15 18 21 24 t/h Fig. 2. Temporal variation of nitrite, nitrate and TN concentration, the value of pH and OD600 for aerobic denitrification of strain CP1. Wu et al., Aerosol and Air Quality Research, 13: 1126–1132, 2013 1130 denitrification efficiency accumulation rate of nitrite OD 100.00% 1.2 60.00% 0.9 40.00% 0.6 20.00% 0.3 OD 600 Rate 80.00% 1.5 0.00% 0 1 3 6 C/N 9 12 15 Fig. 3. The effect of C/N ratios on cell growth and denitrification by strain CP1. Electron Donor Specificity The denitrification electron donor specificity of CP1 was investigated in DM containing the same C/N ratio (12) and initial nitrate concentration (138.5 mg/L) under aerobic conditions. The use of different carbon sources as electron donors affected the denitrification activity of this bacterium (Table 2). Of the carbon sources tested, CP1 removed nitrate by use of from glucose, citrate, succinate with high denitrification efficiency and no nitrite accumulated. However, 23.22% denitrification efficiency and almost halved accumulation of nitrite indicated that acetate was not a suitable carbon source for CP1. There was hardly any removal efficiency when sucrose was used as carbon source. Effect of Temperature on Cell Growth and Denitrification To characterize the effect of varying temperature on CP1 for cell activity and aerobic denitrification, flask cultures were incubated at 20°C, 25°C, 30°C, 37°C, 40°C, 45°C for 24 h under aerobic conditions, respectively. The growth and denitrification efficiency profiles of denitrifier were plotted in Fig. 4. At a temperature of 20°C, the cell activity was inhibited. Therefore, the value of OD600 and denitrification efficiency had only reached 0.391 and 21.79%, respectively, in addition of sizeable proportion of nitrite accumulation. The rise of temperature improved both the growth of strain CP1 and the removal of nitrogen significantly. When the temperature was increased to 30°C, the denitrification efficiency and OD600 quickly increased to a maximum of 97.54% and 1.214, respectively, and nitrite was detected only at extremely low concentrations. When temperature was maintained in a range of 30°C to 40°C, there was no significant change on cell activity. It indicated that the strain CP1 grew well and maintained a high level of denitrification efficiency while temperature varied from 30°C to 40°C. Under the condition of 45°C, the OD600 was slightly lower and the nitrate was almost completely reduced. However, the rate of nitrite accumulation increased rapidly to 85.68%. These phenomena could be explained in that the activity of nitrite reductase was inhibited and thus nitrite was accumulated at this temperature. Effect of Dissolved Oxygen The effect of DO on denitrification efficiency from 138.5 mg/L NO3–-N during a 24-h growth period at 30°C was investigated (Table 3). Studies have shown that the level in DO concentration in any solution is strongly affected by variations in shaking speed (McDaniel et al., 1969; Ho and Chou, 2000; Joo et al., 2005; Qi et al., 2006). With increased shaking speeds there was a corresponding increase in DO concentration. In this study, the adjustments of DO concentration in cultures were made by changing the shaking speed levels. In one group of experiments, the flask was evacuated and pure nitrogen was fully pressurized into the flask before autoclaving. The mouth of flask was sealed with a rubber plug and DO concentration was maintained at essentially zero. The results revealed that the denitrification efficiencies were not affected by DO concentration and the strain maintained denitrification efficiency above 95% when the DO concentration varied from 2.1 to 7.2 mg/L. For the denitrification efficiency, there was not much difference between anoxic condition and aerobic condition. The bacterium performed simultaneous O2 and nitrate respiration. Most studies considered that there exists a strong relationship between DO concentration and denitrification efficiency (Taylor et al., 2009). However, part of existing research (Su et al., 2001) had the distinct conclusion that the existence of oxygen did not inhibite the activity of nitrate reductase and nitrite reductase. The results indicated that the aerobic denitrifier CP1 was with strong adaptability in the application for practical engineering in field of NOx remove from flue gas and denitrification for wastewater under high oxygen conditions. CONCLUSION To explore the characteristic of microorganisms used in the BF system for removal of NOx from flue gas in a coalfired power plant, a dominant aerobic denitrifier of Pseudomonas aeruginosa CP1 was isolated from biofilm in the BF system. The newly isolated strain CP1 had a high denitrifying ability, removing 98.49% of the nitrate in a 24-h period in an aerobic environment, with no nitrite accumulation. From the nitrogen balance, the percentage of nitrogen lost in the flask culture was estimated to be 32.3%, which was presumed to be converted to nitrogen gas. The removal ability was not inhibited by high oxygen conditions Wu et al., Aerosol and Air Quality Research, 13: 1126–1132, 2013 1131 Table 2. The denitrification activity by CP1 using different carbon sources. Carbon source Sucrose Acetate Glucose Citrate Succinate Denitrification efficiency (%) 0.17 ± 0.06 23.22 ± 2.34 87.34 ± 3.39 92.31 ± 4.16 95.82 ± 3.27 Accumulation rate of nitrite (%) 0.66 ± 0.09 9.23 ± 0.14 0.09 ± 0.01 0 0 Table 3. The effect of DO on denitrification activity by CP1. DO (mg/L) 0 2.1 3.3 5.1 7.2 Denitrification efficiency (%) 96.90 ± 1.93 95.12 ± 2.21 95.84 ± 1.79 97.50 ± 2.04 96.92 ± 1.76 Accumulation rate of nitrite (%) 0.03 ± 0.02 0.04 ± 0.01 0.13 ± 0.02 0.13 ± 0.01 0.10 ± 0.01 100.00% 1.4 1.2 80.00% 40.00% 20.00% 0.8 0.6 OD600 Rate 1 denitrification efficiency accumulation rate of nitrite OD 60.00% 0.4 0.2 0.00% 0 20 25 30 37 40 45 T/℃ Fig. 4. The effect of temperature on cell growth and denitrification by strain CP1. and occured best under conditions of C/N ratio 12, 30–40°C using glucose, citrate, succinate as carbon source. These results can be useful information for operating aerobic denitrification process in the BF system engineering application. The CP1 was isolated from BF system for treating NOx and hence, should be suitable for the removal of NOx by means of immobilized cells. The strain was shown to have an excellent denitrifying capability under aerobic conditions. Thus, this strain may also be an attractive candidate for application in high concentration of nitrate and nitrite containing wastewater treatment. ACKNOWLEDGMENTS This research was financially supported by National Natural Science Foundations of China (Grants No. 20777019), Guangdong Provincial Department of Science and Technology Department (Grants No. 2011B090400284 and 2011B010100029). REFERENCES Apel, W.A. and Turick, C.E. (1993). The Use of Denitrifying Bacteria for the Removal of Nitrogen Oxides from Combustion Gases. Fuel 72: 1715–1718. APHA (1992). Standard Methods for the Examination of Water and Wastewater, 18th ed. American Public Health Association, Washington, DC. Barman, S. and Philip, L. (2006). Integrated System for the Treatment of Oxides of Nitrogen from Flue Gases. Environ. Sci. Technol. 40: 1035–1041. Chang, Y.Y., Yan, Y.L., Tseng, C.H., Syu, J.Y., Lin, W.Y. and Yuan, Y.C. (2012). Development of an Innovative Circulating Fluidized-Bed with Microwave System for Controlling NOx. Aerosol Air Qual. Res. 12: 379–386. Ferguson, S.J. (1994). Denitrification and Its Control. Anton. Leeuw. In.t J. G. 66: 89–110. Flanagan, W.P., William, A.A., Barnes, J.M. and Lee, B.D. (2002). Development of Gas Phase Bioreactors for the Removal of Nitrogen Oxides from Synthetic Flue Gas Streams. Fuel 81:1953–1961. Han, S.Q., Bian, H., Feng, Y.C, Liu, A., Li, X.J., Zeng, F. and Zhang, X.L. (2011). Analysis of the Relationship between O3, NO and NO2 in Tianjin, China. Aerosol Air Qual. Res. 11: 128–139. Ho, W.L. and Chou, C.C. (2001). Effect of Carbon and Nitrogen Sources, Sodium Chloride and Culture Conditions on Cytotoxin Production by Salmonella Choleraesuis. Int. J. Food Microbiol. 67: 81–88. Hsieh, L.T., Wang, Y.F., Li, P. and Chen, K.C. (2011). 1132 Wu et al., Aerosol and Air Quality Research, 13: 1126–1132, 2013 Removal of Particle-bound Water-soluble Ions from Cooking Fume Using Bio-solution Wet Scrubber. Aerosol Air Qual. Res. 11: 508–518. Jiang, R., Huang, S., Chow, A.T. and Yang, J. (2009a). Nitric Oxide Removal from Flue Gas with a Biotrickling Filter Using Pseudomonas Putida. J. Hazard. Mater. 164: 432– 441. Jiang, R., Huang, S., Yang, J., Deng, K. and Liu, Z. (2009b). Field Applications of a Bio-trickling Filter for the Removal of Nitrogen Oxides from Flue Gas. Biotechnol. Lett. 31: 967–973. Jin, Y., Veiga, M.C. and Kennes, C. (2005). Bioprocesses for the Removal of Nitrogen Oxides from Polluted Air. J. Chem. Technol. Biotechnol. 80: 483–494. Joo, H.S., Hirai, M. and Shoda, M. (2005). Nitrification and Denitrification in High-strength Ammonium by Alcaligenes Faecalis. Biotechnol. Lett. 27: 773–778. Kesserǚ, P., Kiss, I., Bihari, Z. and Polyák, B. (2003). Biological Denitrification in a Continuous Flow Pilot Bioreactor Containing Immobilizes Pseudomonas Butannovora Cells. Bioresour. Technol. 87: 75−80. Kim, J.K., Park, K.J., Cho, K.S., Nam, S.W., Park, T.J. and Bajpai, R. (2005). Aerobic Nitrification Denitrification by Heterotrophic bacillus Strains. Bioresour. Technol. 96: 1897−1906. Lukow, T. and Diekmann, H. (1997). Aerobic Denitrification by a Newly Isolated Heterotrophic Bacterium Strain TL1. Biotechnol. Lett. 11: 1157–1159. MacFaddin, T.F. (1984). Biochemical Tests for Identification of Medical Bacteria, Williams & Wilkins, Baltimore, MD, p. 36–308. McDaniel, L.E. and Bailey, E.G. (1969). Effect of Shaking Speed and Type of Closure on Shake Flask Cultures. Appl. Environ. Microbiol. 17: 286–290. Murray, R.G.E., Doetsch, N.R. and Robinow, C.F. (1994). Determinative and Cytological Light Microscopy, In Methods for General and Molecular Bacteriology, Gerhardt, P., Murray, R.G.E., Wood, W.A. and Krieg, N.R. (Eds.), American Society for Microbiology Press, Washington, D.C., p. 36. Patureau, D., Bernet, N., Delgenès, J.P. and Moletta, R. (2000). Effect of Dissolved Oxygen and Carbon-nitrogen Loads on Denitrification by an Aerobic Consortium. Appl. Microbiol. Biotechnol. 54: 535–542. Qi, Z., Shinji, T., Shuichiro, M., Phisit, S., Ampin, K. and Kenji, A. (2007). Screening and Characterization of Bacteria That Can Utilize Ammonium and Nitrate Ions Simultaneously under Controlled Cultural Conditions. J. Biosci. Bioeng. 103: 185–191. Reddy, B.S.K., Kumar, K.R., Balakrishnaiah, G., Gopal, K.R., Reddy, R.R., Sivakumar, V., Lingaswamy, A.P., Arafath, S.Md., Umadevi, K., Kumari, S.P., Ahammed Y.N. and Lal, S. (2012). Analysis of Diurnal and Seasonal Behavior of Surface Ozone and Its Precursors (NOx) at a Semi-Arid Rural Site in Southern India. Aerosol Air Qual. Res. 12: 1081–1094. Robertson, L.A. and Kuenen, J.G. (1983). Thiosphaera pantotropha gen. nov. sp. nov., a Facultatively Anaerobic, Facultatively Autotrophic Sulphur Bacterium. J. Gen. Microbiol. 129: 2847–2855. Su, J.J., Liu, B.Y. and Liu, C.Y. (2001). Comparison of Aerobic Denitrification under High Oxygen atmosphere by Thiosphaera pantotropha ATCC 35512 and Pseudomonas stutzeri SU2 Newly Isolated from the Activated sludge of a Piggery Wastewater Treatment System. J. Appl. Microbiol. 90: 457–462. Takaya, N., Catalan-Sakairi M.A.B., Sakaguchi, Y., Kato, I., Zhou, Z. and Shoun, H. (2003). Aerobic Denitrifying Bacteria that Produce Low Levels of Nitrous Oxide. Appl. Environ. Microbiol. 69: 3152–3157. Taylor, S.M., He, Y.L., Zhao, B. and Huang, J. (2009). Heterotrophic Ammonium Removal Characteristics of an Aerobic Heterotrophic Nitrifyingdenitrifying Bacterium, Providencia Rettgeri YL. J. Environ. Sci. 21: 1336–1341. Wang, J.D., Wu, C.Q., Chen, J.M. and Zhang, H.J. (2006). Denitrification Removal of Nitric Oxide in a Rotating Drum Biofilter. Chem. Eng. J. 121: 45–49. Received for review, July 30, 2012 Accepted, November 15, 2012