article - Aquatic Invasions
advertisement

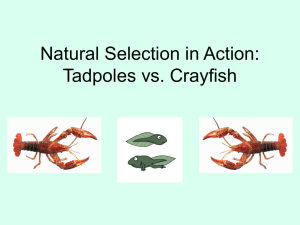
Aquatic Invasions (2014) Volume 9, Issue 1: 47–58 doi: http://dx.doi.org/10.3391/ai.2014.9.1.04 © 2014 The Author(s). Journal compilation © 2014 REABIC Open Access Research Article First evidence of microfungal “extra oomph” in the invasive red swamp crayfish Procambarus clarkii Laura Garzoli 1 * , Daniele Paganelli 2 , Marinella Rodolfi 1 , Dario Savini 2 , Mattia Moretto 2 , Anna Occhipinti-Ambrogi 2 and Anna Maria Picco 1 1 2 Lab. of Mycology, DSTA-Department of Earth and Environmental Sciences, University of Pavia, Via S. Epifanio 14, 27100 Pavia, Italy Lab. of Ecology, DSTA-Department of Earth and Environmental Sciences, University of Pavia, Via S. Epifanio 14, 27100 Pavia, Italy E-mail: laura.garzoli01@gmail.com (LG), daniele.paganelli@unipv.it (DP), marinella.rodolfi@unipv.it (MR), dario.savini@unipv.it (DS), mattia.moretto01@universitadipavia.it (MM), anna.occhipinti@unipv.it (AOA), annamaria.picco@unipv.it (AP) *Corresponding author Received: 17 July 2013 / Accepted: 10 January 2014 / Published online: 3 February 2014 Handling editor: Chris Chucholl Abstract This paper represents the first attempt to study the microfungal flora contained in the digestive system of the most widespread aquatic invasive invertebrate in the world: Procambarus clarkii (Cambaridae, Decapoda). Understanding its bioinvasion, in terms of ecological risk and environmental impact, requires a multidisciplinary approach that considers consequences on all levels, from macroscopic to microscopic. In this study, we investigated both the population dynamics and the dietary habits of the red swamp crayfish captured within a natural biotope in Northern Italy. The diet was mainly based on plant detritus. The analysis of microfungal flora in the crayfish stomach revealed a fairly constant composition, unrelated to season and diet. Since most of the fungi were associated with detritus and some species were particularly frequent, we formulate the hypothesis that the fungi may be selected to decompose plant material in the digestive tract, thus providing a source of energy to the crayfish. Procambarus clarkii is also shown to be a potential vector of plant diseases as some of the 45 isolated fungal taxa are potentially phytopathogenic. Key words: population study, diet, microfungi, digestive system, wet woodland biotopes, Northern Italy Introduction The red swamp crayfish Procambarus clarkii (Girard, 1852) is one of the top 27 alien species of animals to have been introduced into Europe for aquaculture (Savini et al. 2010), and is considered to be one of the most dangerous species in terms of ecological risk and environmental impact (Geiger et al. 2005; Gherardi and Acquistapace 2007; Tricarico et al. 2010). Although P. clarkii is a North American native species (Huner 1988), due to its outstanding performances in aquaculture productivity, it was legally introduced into southern Spain in 1973, and later illegally introduced into most other European countries between 1970 and 1990 (SoutyGrosset et al. 2006). Many biological traits, such as the capability to live out of water for long periods (Gherardi and Barbaresi 2007), a high reproductive potential (Aquiloni et al. 2010; Barbaresi and Gherardi 2006; Savini 2007), and tolerance to environmental pollution (Aquiloni et al. 2011), are responsible for the rapid spread of crayfish in the wild. High motility and burrowing behaviour make this species one of the most successful fugitives from aquaculture facilities (Gherardi 2006). Nowadays, P. clarkii is widespread in Western Europe, Africa, Asia and North America; the only continents where the crayfish has never been recorded are Australia and Antarctica (Gherardi 2006). In Italy, Procambarus clarkii appeared in the wild for the first time in 1989 in the river Banna, a tributary of the Po river, after escaping from an experimental farm (Delmastro 1992); its distribution currently includes the majority of the northern and central regions, as well as the 47 L. Garzoli et al.C. islands (Gherardi et al. 1999a; Chiesa et al. 2006; Savini and Occhipinti 2008; Scalici et al. 2009; Aquiloni et al. 2011), and it has also been recorded on several occasions in southern Italy (Morpugo et al. 2010). A comprehensive review of the impact of this crustacean in wetlands was provided by Geiger et al. (2005). Lodge et al. (2012) also presented a significant review on the categories of ecosystem services potentially affected by nonindigenous crayfish, including P. clarkii. Overall, there are more than 40 peer reviewed papers that have proved and in some cases quantified the environmental impact of P. clarkii (Savini et al. 2010). Nevertheless, understanding the consequences of the introduction of an alien species into a new environment involves the functioning of the ecosystem as a whole from macroscopic to microscopic level, and therefore requires a multidisciplinary approach that transcends the boundaries of single area of expertise. One of the key factors of the success of P. clarkii is its capability “to make a virtue of necessity”: crayfish are generally considered to be polytrophic feeders that can successfully adapt their diet to food availability (Gutiérrez-Yurrita et al. 1998; Momot 1995; Correia 2003; Alcorlo et al. 2004). One of the less considered aspects of P. clarkii bioinvasion is its interaction with the microbial community in freshwater ecosystems. As a consequence of its feeding behaviour, P. clarkii needs enzymes that can break down different types of food: this role could be related to microorganism activity. Fungi and bacteria associated with leaves can produce cellulosases, xilanases, pectinases and other enzymes that aquatic invertebrates may use to digest complex substrates (Graça 2001). In particular, microfungi produce a mosaic of heterogeneous substrates on leaf litter, which are variable in time and space, and available for shredders (Rossi 1983). Besides enhancing substrate palatability, fungi themselves represent a food source and are known to be a target for aquatic invertebrates. This double role of microfungi in the feeding strategies of aquatic invertebrates has been clearly demonstrated by Graça et al. (1993) for Asellus aquaticus (Linnaeus, 1758) and Gammarus pulex Linnaeus, 1758. In the first case, the crustacean selectively consumed fungal mycelia, while in the second, leaf material was chosen depending on fungal colonisation. Although it is generally assumed that crayfish mainly feed on conditioned leaves, few authors have clearly demonstrated this preference in crayfish: only 48 Momot et al. (1978) demonstrated that young Orconectes virilis did not eat plant material but rather appeared to scrape it clean off periphytic growths. Moreover, symbioses between aquatic invertebrates and their stomach microflora play a fundamental role in digestive processes. In 1993, Harris provided a comprehensive synthesis of available studies on the presence, nature and role of gut microflora in aquatic invertebrates. Nevertheless, published data on crayfish gut microflora and its biochemical activity are scarce or limited to a few species (Mickënienë and Šyvokienë 1996). In the present study, we investigated a small population of P. clarkii in a natural area of the Ticino Regional Park (North Italy), mainly focusing on its diet and its digestive system fungal microflora. The latter aspect has not been considered until now, but may facilitate the success of P. clarkii in a new environment. Methods Study area The Ticino river is one of the main tributaries of the Po river, flowing in the southern part of Lombardy (NW Italy), along a valley measuring 110km long and 7km wide. Although this river plain is characterised by intensive agriculture and anthropogenic activities, numerous natural reserves (partial and integral) occupy a large part of the valley (about 91,000ha). Our study site, called “I Geraci” (WGS84 45°16'34.46"N, 8°59' 0.14"E), measures 90ha and is situated within the Ticino Regional Park, one of such natural reserves (Figure 1). The Geraci area is located on the hydrological left side of the Ticino and, due to its proximity to the river, is often overflooded. This area is a typical natural biotope of the Southern Po Plain, surrounded by agricultural fields and small villages; it is composed of numerous wetlands, ponds and small rivers, mainly concentrated in the central part of a dense wood, which is quite impenetrable and isolated. The water temperature of the wetlands varied from 0°C in winter to over 28°C in summer, a typical range in the Southern Lombardy Plain. Population abundance of Procambarus clarkii To investigate the population of P. clarkii, a weekly sampling program was carried out from June First evidence of microfungal “extra oomph” in Procambarus clarkii Figure 1. Geraci: a 90-hectare natural area (boundaries in a dotted line) located on the hydrological left side of the Ticino river within the Ticino Regional Park (NW Italy). Circles indicate sampling stations (T1 to T13). Digital orthophoto of the studied area derived from the Lombardy Region Orthophoto 2003 (scale of the frames: 1:40000, average altitude of flight: 6000m). 2009 to May 2010 using 13 double entrance crayfish “Trappy Tetra” made of polypropylene plastic (length 520mm, diam. 210mm and mesh size 20mm), always set in the same location within the sampling area (Figure 1). Pig liver was used as bait to attract crayfish (50g per trap per week). These traps are highly selective for crayfish, thus minimising by-catches of fish, reptiles and small mammals. For each captured specimen, sex, weight and total body length (from the tip of the rostrum to the tip of the telson) were recorded, as suggested by Scalici and Gherardi (2007); the crayfish were then frozen at -20°C. Average monthly catches per unit effort (AVCPUE) was used as a standardised index of abundance (Richards and Schnute 1986; Hinton and Maunder 2003; Savini 2007; Savini and Occhipinti 2008; Savini et al. 2008). Diet composition and determination of stomach microfungal flora For each sampling month, the food choices and stomach mycoflora of five crayfish, chosen at random, were investigated. The stomach content was analysed following the procedure proposed by Correia (2003). The percentage of occurrence of each food category was estimated: detritus, plants (green plants, seeds, remains of seeds, and others) and animals. Crayfish with scars, abrasion or empty stomachs were excluded from the analysis. This selective choice was due to the fact that crayfish with impairments are less active and more stressed than others (Figiel and Miller 1995; Kraus-Epley and Moore 2002; Bergman et al. 2003). Stomachs were excised under a laminar flow hood to prevent external contamination, then a smear of stomach content was plated into different media using a sterile loop: the generic medium PDA (Potato Dextrose Agar: 200g potato, 20g dextrose, 15g agar, 1000ml distilled water); the nutrient medium MEA (Malt Extract Agar: 30g malt extract, 15g agar, 5g peptone, 1000ml distilled water); and the specific medium for the culture diagnosis of Aphanomyces astaci Schikora, 1906, causal agent of the crayfish plague, GOYA (Glucose Oxolinic Yeast extract Agar: 12g agar, 1g yeast extract, 5g glucose, 10mg Oxolynic acid, 1000ml distilled water), which is also favourable for the growth of both fungal parasites 49 L. Garzoli et al.C. and saprophytes, as reported by Alderman and Polglase (1986). Furthermore, the stomach volume was estimated: a random sample (10 crayfish) of extracted crayfish stomachs was closed with pincers, filled with water up to the maximum capacity, and then a computerised system of image analysis approximated each stomach to a sphere (approximate volume=2ml). In order to facilitate the isolation of slow growing mitosporic fungi, a 1:10 dilution was performed and plates were incubated at 25°C. Examination of plates was continued for up to four weeks to detect the presence of sporulating fungi. Initial detection of fungal structures was carried out by means of stereomicroscope ( 1050) and strains were identified on the basis of micro-morphological analysis by means of specific taxonomical keys (Ellis 1971, 1976; Samson 1974; Hermanides-Nijhof 1977; Pitt 1979; Domsch et al. 1980; Sutton 1980; Nelson et al. 1983; Klich 2002; Samson et al. 2004). Although the aim of this study was to investigate fungal flora, the amount of both Bacteria and Actinomycetes (in CFU) for each plate is also provided. Lastly, microfungal strains were collected as pure cultures onto the generic microfungi medium PDA. Statistical analyses All the statistical analyses were performed using PRIMER 5.0 (Plymouth Routines In Multivariate Ecological Research, Clarke and Warwick 1994) and MINITAB 15.0 (Minitab Inc. 2007) software packages. A G-test was used to evaluate the sex-ratio within the population and the diet composition. A one-way ANOVA was performed to test seasonal differences in crayfish diet. We run a Tukey post hoc to identify seasonal differences in crayfish diet. Furthermore, the number of specimens captured in each month of the survey was compared using a Kruskal-Wallis test, and a General Linear Model (GLM) was applied in order to highlight biometric differences. Differences in microfungal community composition between seasons were tested with an ANOSIM test based on the Bray-Curtis similarity index; BIO-ENV analysis was carried out to correlate stomach content with the recovered microfungal flora (Clarke and Warwick 1994). 50 Results Population abundance and structure A total of 692 P. clarkii specimens were captured over the one-year survey period: 490 males and 202 females (sex ratio of 2.42 males to 1 female). The only time that this ratio was not respected was in late winter (February, March), when the number of captured females was slightly higher than males. A G test confirms this statistical difference between sexes (G=36.93; DF=11; p=0.0001). The highest mean CPUE value of P. clarkii was registered in summer (AVCPUE in July 2009=2.67±2.09), while the lowest was registered in winter (AVCPUE in February 2010=1.3±0.67) (Figure 2). Differences between CPUE per month were tested by means of a K-Wallis test and results showed a significant difference (H=22.06; DF=11; p=0.048). The overall mean length of specimens was 98.77±11.36mm, the mean length of male specimens was 97.41±10.94mm, and the mean length of female specimens was 100.86±11.98mm (Figure 3). A General Linear Model (GLM) was used to test biometric differences between the size of males and females observed during the year. The results confirmed that there is a significant statistical difference between months (DF=11; F=2.9; p<0.05). Female specimens were significantly larger than male specimens (DF=1; F=6.9; p< 0.05) and there was no interaction between variables (DF = 11; F = 1.3; p>0.05). A size-class distribution analysis over the whole year showed that the most frequently captured specimens measured between 90-99mm and 100109mm. Smaller specimens (size class between 40mm and 49mm) were captured during late winter-early spring (February-March 2010) (Figure 4). Stomach content analysis In the Geraci area, the diet of P. clarkii mainly consisted of detritus (55.56% of the total) and plant material (43.22%); animal consumption was low (1.22%) (G: 2845.48; DF=88; p<0.001). The highest dietary component was detritus in spring and summer (76% and 61.5% respectively), and plants in autumn and winter (72% and 52% respectively) (G: 58.96; DF=3 p<0.001) (Figure 5). First evidence of microfungal “extra oomph” in Procambarus clarkii Figure 2. Monthly Catches Per Unit Effort (CPUE), a standardised index to define the abundance of P. clarkii from June 2009 to May 2010. The whiskers extend outwards to indicate the lowest and highest values in the data set (excluding outliers). Extreme values, or outliers, are represented as asterisks. Figure 3. Box-plot of the total body length of P. clarkii female (white) and male (grey) specimens collected in the Geraci area from June 2009 to May 2010. The whiskers extend outwards to indicate the lowest and highest values in the data set (excluding outliers). Extreme values, or outliers, are represented as asterisks. The seasonal differences in percentages of plants found in the stomachs were statistically significant (1-way ANOVA: DF=3; F=7.94; p<0.001); the same analysis was performed using the percentage of detritus as a variable and differences between seasons were again statistically significant (DF=3; F=7.14; p<0.005). Tukey post hoc tests were performed in order to highlight seasonal differences in crayfish diet. Autumn significantly differs from spring and summer in terms of plant and detritus consumption (Table 1). Furthermore, in order to highlight possible differences in diet composition between sexes, a General Linear Model (GLM) was performed: results supported the hypothesis that there were no significant differences between sexes (Detritus: DF=1, F=1.92, p>0.05; Plant material: DF=1, F=1.66, p>0.05; Animal: DF=1, F=0.16, p>0.05). Microfungal stomach flora A total of 45 taxa were found in the stomachs of the 45 examined specimens: Table 2 shows the total frequency (i.e. the percentage of P. clarkii individuals in which the taxon was observed over the total number of examined specimens). Microfungal specimens were divided into two categories, Dematiaceous, i.e. containing melanins in cell walls, and non-Dematiaceous, according to Ellis (1971, 1976). The majority of observed taxa (80%) consisted of non-Dematiaceous fungi, while 20% consisted of Dematiacea species. Mycelia sterilia and yeasts were detected with the highest frequencies (73% and 64% respectively), followed by Penicillium sp. (62%), Talaromyces flavus (42%), Trichoderma sp. (33%), Mucor sp. (22%), Cladosporium sp. (18%), Acremonium sp. (11%); the remaining taxa were found with a lower 51 L. Garzoli et al.C. Figure 4. Size-class distribution of P. clarkii male specimens (light grey) and female specimens (dark grey). a) June 2009, b) July 2009, c) August 2009, d) September 2009, e) October 2009, f) November 2009, g) December 2009, h) January 2010, i) February 2010, j) March 2010, k) April 2010, l) May 2010. Table 1. Results of Tukey Simultaneous tests. The animal category was not considered as it was inconsistent. Seasons autumn vs summer autumn vs winter autumn vs spring summer vs winter summer vs spring winter vs spring autumn vs summer autumn vs winter autumn vs spring summer vs winter summer vs spring winter vs spring 52 Seasonal differences in percentages of plants Tukey test p value -3.36 0.0096 -2.00 0.2048 -4.66 0.0002 1.35 0.5357 -1.30 0.5666 -2.65 0.0542 Seasonal differences in percentages of detritus 3.47 0.0070 2.11 0.1673 4.33 0.0006 -1.36 0.5316 0.857 0.8269 2.217 0.1379 Statistical significant ** NS *** NS NS NS * NS *** NS NS NS First evidence of microfungal “extra oomph” in Procambarus clarkii Table 2. Frequency of occurrence (%) of microfungal taxa in the stomach samples of P. clarkii in relation to capture seasons. Percentages are in relation to the total sample (TS); summer 2009 (S09); autumn 2009 (A09); winter 2009/2010 (W09/10); spring 2010 (SP10). Taxa TS S09 A09 W09/10 Sp10 Mycelia sterilia Yeasts Penicillium sp. Talaromyces flavus Trichoderma sp. Mucor sp. Acremonium sp. Cladosporium sp. Aspergillus fumigatus Fresen. (1863) A. brasiliensis Varga, Frisvad & Samson 2007 Aspergillus sp. Pestalotiopsis guepinii (Desm.) Steyaert (1949) Rhizopus stolonifer (Ehrenb.) Vuill. 1902 Arthrinium sp. Cladosporium cladosporioides (Fresen.) G.A. de Vries Mucor hiemalis Wehmer (1903) Phoma sp. Arthrinium phaeospermum (Corda) M.B. Ellis (1965) Aureobasidium pullulans var. pullulans (de Bary) G. Arnaud 1918 Cladosporium chlorocephalum (Fresen.) E.W. Mason and M.B. Ellis (1953) Emericellopsis sp. Khuskia oryzae H.J. Huds. (1963) Paecilomyces sp. Penicillium verrucosum Dierckx (1901) Scopulariopsis sp. Sordaria fimicola (Roberge ex Desm.) Ces. and De Not. (1863) Absidia fusca Linnem. (1936) A. glauca Hagem (1908) Alternaria alternata (Fr.) Keissl. 1912 Acremonium persicinum (Nicot) W. Gams (1971) Aspergillus clavatus Desm. (1934) A. flavus Link (1809) A. glaucus (L.) Link (1809) A. versicolor (Vuill.) Tirab. (1908) Aureobasidium pullulans var. melanogenum Herm.-Nijh (1977) Cephalotrichum microsporum (Sacc.) P.M. Kirk (1984) Chaetomium sp. Coniella sp. Fusarium sp. Hemicarpenteles ornatum (Subram.) Arx (1974) Mortierella turficola Y. Ling (1930) Mortierella sp. Oidiodendron flavum Svilv. (1941) Paecilomyces farinosus (Holmsk.) A.H.S. Br. and G. Sm. (1957) P. inflatus (Burnside) J.W. Carmich. (1962) P. lilacinus (Thom) Samson (1974) Actinomycetes Bacteria 73 64 62 42 33 22 18 18 11 11 9 9 9 7 7 7 7 4 4 4 4 4 4 4 4 4 2 2 2 2 2 2 2 2 2 2 2 2 2 2 2 2 2 2 2 2 42 93 70 70 60 90 30 40 30 50 0 10 0 10 10 0 0 0 20 10 10 0 0 0 10 0 0 20 10 0 0 0 0 0 0 0 0 0 10 10 0 0 0 10 0 10 10 10 70 100 70 30 40 20 10 10 10 10 0 40 20 10 10 0 30 0 0 0 10 10 20 0 0 0 20 0 0 0 10 0 0 0 0 0 0 10 0 0 10 0 0 0 0 0 0 0 50 100 70 80 80 20 30 30 10 20 30 0 10 20 10 20 0 10 0 0 0 0 0 10 0 0 0 0 0 0 0 0 10 10 0 0 10 0 0 0 0 0 10 0 0 0 0 0 30 90 80 73 67 40 53 13 20 0 13 0 7 0 7 7 0 13 7 7 0 7 0 7 7 13 0 0 0 7 0 7 0 0 7 7 0 0 0 0 0 7 0 0 7 0 0 0 27 87 frequency than 10%. Bacteria and Actinomycetes were also observed throughout. An ANOSIM test was performed in order to highlight differences in the microfungal flora community between seasons: results suggested there were no significant differences between seasons (R=0.207; p: 0.001) and the highest taxa richness was recorded in spring 2010. Furthermore, a BIO-ENV analysis correlating stomach content with its microfungal flora showed a low negative correlation between variables (p=-0.026). Discussion Studies involving multidisciplinary expertise are of fundamental importance to understand environmental alterations that can lead to changes in population dynamic properties. Moreover, they can be used as indicators of different kinds of stressors that have never been considered before. Understanding P. clarkii population dynamics is a useful way of building effective management plans to control this dangerous alien species. 53 L. Garzoli et al.C. Figure 5. Seasonal composition of the P. clarkii diet in the Geraci area (5 analysed crayfish/month). Lodge et al. (2012) stated that it is fundamental to quantify the impact of invasive crayfish through metrics of direct relevance when making provisions and regulating ecosystem services, both cultural and monetised. Therefore, the cost of one year of systematically trapping P. clarkii was evaluated in order to better inform management decisions. The following factors were taken into consideration: the hourly rate paid to each operator (€10/hour), bait costs (€5/kg), trap costs (€15/trap), and transport mobility expenses. The total budget required to control the invasive crayfish in a small area such as Geraci was about €5000: €192 for baits, €300 for traps, €52 for complementary material, €1411 for mobility (car fuel and transfer costs), and €2880 for two operators. Intensive systematic trapping, coupled with other management strategies, such as predatory fishing, can be effective in controlling the crayfish population in invaded and closed ecosystems (Frutiger et al. 1999; Hein et al. 2006), but it is problematic when used for population control in an open environment (as in our case study) where immigration/emigration phenomena from neighbouring environments affect population size (Barbaresi and Gherardi 2000). Catches are also influenced by temperature, crayfish foraging and reproduction activity, as well as body size (Correia 2001; Chucholl 2011). Large specimens, especially males, being more mobile than juveniles, are more likely to be captured, whereas juvenile specimens are both “trap-shy” and small enough to escape. In our study, the highest number of crayfish was collected in summer when P. clarkii is more active, and the lowest number in winter, when 54 the population is mainly composed of juvenile specimens. Moreover, crayfish collected over the whole year were always longer than 40mm, thus post-juvenile specimens. This suggests that trapping has limited effectiveness in red swamp population control. As regards the population structure, we found a sex ratio of 2.42 males to 1 female. According to previous studies conducted in central Italy, a stable population of P. clarkii has a sex ratio of 1:1 (Gherardi et al. 1999a; Scalici and Gherardi 2007). However, as various authors have stated, a higher proportion of males can depend on the capture method used, as traps are mainly maleselective (Ackefors 1999; Ilhéu et al. 2003; Savini 2007; Chucholl 2011). Overall, our data confirm the presence of a stable population of P. clarkii in the Geraci area. The highest density of crayfish was registered in the centre of the Geraci area (T5 to T8). This area is characterised by small shallow wetlands, with steep embankments in which P. clarkii specimens dig their burrows. The bottom of the ponds is rich in detritus and plant material, in accordance with our findings on the feeding habits of P. clarkii. As food availability is site-specific, knowledge about the P. clarkii diet can help to understand its effect on trophic food webs. Our results confirm that freshwater crayfish are opportunistic and omnivorous feeders (Lodge and Hill 1994; Momot 1995; Gutiérrez-Yurrita et al. 1998). Correia (2003) found that P. clarkii mainly feeds on plants during spring and summer, while it prefers an animal diet during winter. Our data, however, did not confirm this trend: stomach content mainly consisted of plant material during autumn and winter, and of detritus (i.e. muddy/sand inorganic matter mixed with decomposed organic matter) during spring and summer. The nutritional value of detritus is mainly linked to the microbial layer surrounding the sediment particles (Hall and Meyer 1998). The absence of aquatic vegetation may also have affected the crayfish diet. The predominance of plant matter in autumn is probably linked to the large quantity of dead leaves that fall to the ground and into the water at the beginning of this season. Thus, our findings confirm that P. clarkii can shift its diet in relation to food availability. In our case study, the animal category was not included in the red swamp adult diet; animal prey was less representative in the diet than expected: this could be explained by the fact that the traps proved to be adult-selective. In fact, small crayfish First evidence of microfungal “extra oomph” in Procambarus clarkii are mainly carnivorous, while larger individuals are primarily herbivorous; animal-based food is much more important for young, rapidly growing juveniles than for adults (Correia 2003). In recent years, there has been a growing interest in the relationship between P. clarkii and environmental microorganisms (Dörr et al. 2011, 2012a,b). Only a few studies are available on microflora in the digestive tract of P. clarkii (Gherardi et al. 1999b; Khalil et al. 2009), none of which focus on fungi. Microorganisms can enter the crayfish stomach not only as food: in particular fungi, which can be highly related to their growing substrates (e.g. saprotrophs can obtain their nutrients from dead organisms, while epi- and endophytes can only survive in association with living plants). As a consequence, seasonal variations in microbial communities in the stomach according to diet were expected. Nevertheless, seasonal differences in the microfungal community were not consistent, as the ANOSIM test confirms. Three partially alternative/complementary explanations can fit our results: 1) Homogeneity of diet. Fungi are accountable for recycling components of dead plants. In our site, detritus is mainly composed of dead plant fragments. We can hypothesise that microfungal assemblages linked to plant material are not very different from those linked to detritus; some of the very common taxa found in all seasons are indeed common saprobes, frequently associated with soil and litter, such as Mucor sp., Rhizophus stolonifer, Thrichoderma sp., and Talaromyces flavus. Other detected fungi are linked to plant hosts/plant material: the widespread taxa Cladosporium sp. and Penicillium sp., and the less common Pestalotiopsis guepinii, the causal agent of the hazelnut twig blight (Türkkan et al. 2011). This result highlights an aspect related to red swamp crayfish bioinvasion: a high population density of P. clarkii could be a vector of introduction not only of animal pathogens (the most famous being Aphanomyces astacii) but also of plant pathogens, whose spores can survive in the digestive tract of crayfish when they reach new environments. 2) Procambarus clarkii selection of food source. It is well known that shredders can selectively feed on leaves that are colonised by microorganisms, in particular fungi (Graça et al. 1993). Not only can fungi increase the palatability of leaves by promoting the transformation of inedible plant material into edible compounds, but they are also a better food source than leaves, in so far as they possess a higher nutritional value (mostly in terms of nitrogen content) (Graça 2001). However, although food choice experiments indicate that most soil animals prefer fungi with dark pigmented hyphae and/or conidia (=Dematiacea) (Maraun et al. 2003), our findings do not support literature data, as the majority of observations are non-Dematiaceous fungi. The preference of Dematiaceous fungi by aquatic invertebrates has been partially related to the higher nutritional value of melanised hyphae. Common species of non-Dematiaceous fungi also possess strong enzymes, such as chitinases in Trichoderma, Penicillium, Paecilomyces and Mortierella, which are usually avoided by arthropods (Maraun et al. 2003). In our study, Penicillium and Trichoderma were found with the highest frequency. This could lead to the third hypothesis. 3) Although less reliable, a fungal selection within the P. clarkii stomach cannot be excluded when attempting to explain the homogeneity of our data-set on fungal assemblages. Several authors point out the effect of the red swamp crayfish herbivory (Vasconcelos et al. 2001; Rodriguez et al. 2005; Anastácio et al. 2005; Savini and Occhipinti 2008). As a consequence, it needs enzymes that degrade complex polymers such as cellulose. Acquired microbial cellulases have been reported among freshwater invertebrates by Bärlocher (1982) and Sinsabaugh et al. (1985). In 1964, Yokoe and Yasumasu demonstrated a considerable cellulasic activity in hepatopancreatic microsomal fractions (i.e. subcellular fraction of gland homogenate) of P. clarkii, but this activity was not related to Gram negative bacilli found in the stomach of the specimens they analysed. Gherardi et al. (1999b) also found a cellulosedegrading bacteria in its digestive tract. Nevertheless, fungal production of endogenous cellulases has never been considered. Many of the isolated fungi found in our study are reported in literature to produce a wide range of hydrolases that digest polymers such as cellulose, lignin and chitin, as is the case with Talaromyces flavus, a species common in soil and in organic substrates. This species is able to degrade starch and cellulose and to produce antibiotic metabolites (such as vermiculine) that are active against bacteria, streptomycetes and protozoans (Domsch et al. 1980). Other taxa that are very common plant parasites and ubiquitous in soil, such as Alternaria alternata and Fusarium sp., were found with low frequencies (Domsch et al. 1980). 55 L. Garzoli et al.C. On the basis of these observations, our findings could be compatible with the selection of fungal assemblage in crayfish stomachs. One of the severe constraints in highlighting arthropod host/fungi interaction is the difficulty obtaining axenic culture of gut fungi that can be used in laboratory infection trials (Kimura et al. 2002). This study is a starting point in this direction, as the microfungal strains are being maintained in pure culture. In conclusion, our data show that the detected mycoflora is not related to season or diet. We hypothesised that the lack of differences may be related to: 1) a relative homogeneity of food sources, 2) a selection of food source more or less colonised by fungi, 3) a selection within the P. clarkii stomach. The third hypothesis can lead to two main conclusions: 1) studies on production of enzymes such as cellulase from endogenous mycoflora should be encouraged in order to better understand their role in P. clarkii bioinvasion; 2) the selection of fungal propagules in the digestive tract of crayfish and combined propagation of such microorganisms in excrements deserve particular attention, since an invasive species that also behaves as a vector of parasites is a further risk in terms of “pathogen pollution” (Picco et al. 2011). Further studies are needed to understand the functional role of fungi in the digestive system and their importance in the invasion process. Acknowledgements We would like to dedicate this paper to the memory of Francesca Gherardi, who recently and prematurely passed away. She was a worldwide renowned leading scientist in the field of biological invasions and a pioneer in the research on P. clarkii in Italy and Europe. Some of us have had the privilege of collaborating with her and will never forget her scientific value, modesty, and ladylike, generous manner. We gratefully thank Charlotte Buckmaster for revising the English text. We wish to thank Prof. Giuseppe Bogliani for his precious support to fieldwork logistic. Our special thanks to Dr. Christoph Chucholl, Dr. Frances Lucy and two anonymous reviewers for their constructive comments and suggestions on the manuscript. References Ackefors H (1999) The positive effects of established crayfish introductions in Europe. In: Gherardi F, Holdich DM (eds), Crayfish in Europe as alien species. How to make the best of a bad situation. A. A. Balkema, Rotterdam, pp 49–61 Alcorlo P, Geiger W, Otero M (2004) Feeding preferences and food selection of the red swamp crayfish (Procambarus clarkii) in habitats differing in food item diversity. Crustaceana 77(4): 435–453, http://dx.doi.org/10.1163/15685400 41643283 56 Alderman DJ, Polglase JL (1986) Aphanomyces astaci: isolation and culture. Journal of Fish Diseases 7: 401–405, http://dx.doi.org/10.1111/j.1365-2761.1984.tb01205.x Anastácio PM, Parente VS, Correia AM (2005) Crayfish effects on seeds and seedlings: identification and quantification of damage. Freshwater Biology 50: 697–704, http://dx.doi.org/ 10.1111/j.1365-2427.2005.01343.x Aquiloni L, Tricarico E, Gherardi F (2010) Crayfish in Italy: distribution, threats and management. International Aquatic Research 2: 1–14 Aquiloni L, Martin MP, Gherardi F, Diéguez-Uribeondo J (2011) The North American crayfish Procambarus clarkii is the carrier of the oomycete Aphanomyces astaci in Italy. Biological Invasions 13: 359–367, http://dx.doi.org/10.1007/ s10530-010-9828-2 Barbaresi S, Gherardi F (2000) The invasion of the alien crayfish Procambarus clarkii in Europe, with particular reference to Italy. Biological Invasions 2: 259–264, http://dx.doi.org/10.102 3/A:1010009701606 Barbaresi S, Gherardi F (2006) Experimental evidence for homing in the red swamp crayfish, Procambarus clarkii. Bulletin Français de la Pêche et de la Pisciculture 380–381: 1145– 1154, http://dx.doi.org/10.1051/kmae:2006017 Bärlocher F (1982) The contribution of fungal enzymes to the digestion of leaves by Gammarus fossarum Koch (Amphipoda). Oecologia 52: 1–4, http://dx.doi.org/10.1007/BF00349003 Bergman DA, Kozlowski CP, McIntyre JC, Huber R, Daws AG, Moore PA (2003) Temporal dynamics and communication of winner–effects in the crayfish, Orconectes rusticus. Behaviour 140(6): 805–825, http://dx.doi.org/10.1163/156853903 322370689 Chiesa S, Scalici M, Givertin G (2006) Occurrence of allochthonous freshwater crayfishes in Latium (Central Italy). Bulletin Français de la Pêche et de la Pisciculture 380–381: 883–902, http://dx.doi.org/10.1051/kmae:2006029 Chucholl C (2011) Population ecology of an alien “warm water” crayfish (Procambarus clarkii) in a new cold habitat. Knowledge and Management of Aquatic Ecosystems 401: 29, http://dx.doi.org/10.1051/kmae/2011053 Clarke KL, Warwick RM (1994) Change in marine communities: an approach to statistical analysis and interpretation. Plymouth Marine Laboratory, 176 pp Correia AM (2001) Seasonal and intraspecific evaluation of predation by mammals and birds on the introduced red swamp crayfish Procambarus clarkii (Crustacea, Cambaridae) in a freshwater marsh (Portugal). Journal of Zoology 255: 533–541, http://dx.doi.org/10.1017/S0952836901001625 Correia AM (2003) Food choice by the introduced crayfish Procambarus clarkii. Annales Zoologici Fennici 40: 517–528 Delmastro GB (1992) Sull'acclimatazione del gambero della Louisiana Procambarus clarkii (Girard, 1852) nelle acque dolci italiane (Crustacea: Decapoda: Cambaridae). Pianura 4: 5–10 Domsch KH, Gams W, Anderson TH (1980) Compendium of soil fungi. Academic Press, London, 865 pp Dörr AJM, Rodolfi M, Scalici M, Elia AC, Garzoli L, Picco AM (2011) Phoma glomerata, a potential new threat to Italian inland waters. Journal for Nature Conservation 19(6): 370– 373, http://dx.doi.org/10.1016/j.jnc.2011.06.006 Dörr AJM, Elia AC, Rodolfi M, Garzoli L, Picco AM, D'Amen M, Scalici M (2012a) A model of co-occurrence: segregation and aggregation patterns in the mycoflora of the crayfish Procambarus clarkii in Lake Trasimeno (central Italy). Journal of Limnology 71(1): 135–143, http://dx.doi.org/10.4081/ jlimnol.2012.e14 Dörr AJM, Rodolfi M, Elia AC, Scalici M, Garzoli L, Picco AM (2012b) Mycoflora on the cuticle of the invasive crayfish Procambarus clarkii. Fundamental and Applied Limnology 180 (1): 77–84, http://dx.doi.org/10.1127/1863-9135/2012/0175 First evidence of microfungal “extra oomph” in Procambarus clarkii Ellis MB (1971) Dematiaceous Hyphomycetes. CAB, Kew, Surrey, England, 594 pp Ellis MB (1976) More Dematiaceous Hyphomycetes. CAB, Kew, Surrey, England, 493 pp Figiel CR Jr, Miller GL (1995) The frequency of chela autotomy and its influence on the growth and survival of the crayfish Procambarus clarkii (Girard, 1852) (Decapoda, Cambaridae). Crustaceana 68(4): 472–483, http://dx.doi.org/10.1163/ 156854095X00683 Frutiger A, Borner S, Büsser T, Eggen R, Müller R, Müller S, Wasmer H R (1999) How to control unwanted populations of Procambarus clarkii in Central Europe. Freshwater Crayfish 12: 714–726 Geiger W, Alcorlo P, Baltanás A, Montes C (2005) Impact of an introduced Crustacean on the trophic webs of Mediteranean wetlands. Biological Invasions 7: 49–73, http://dx.doi.org/10. 1007/s10530-004-9635-8 Gherardi F (2006) Crayfish invading Europe: the case study of Procambarus clarkii. Marine and Freshwater Behaviour and Physiology 39: 175–191, http://dx.doi.org/10.1080/10236240600 869702 Gherardi F, Baldaccini GN, Barbaresi S, Ercolini P, De Luise G, Mazzoni D, Mori M (1999a) The situation in Italy. In: Gherardi F, Holdich DM (eds) Crayfish in Europe as Alien species. How to make the best of a bad situation? Balkema, Rotterdam, pp 107–128 Gherardi F, Acquistapace P, Salvi G, Mastromei G (1999b) Cellulolytic activity in the hindgut of crayfish. Crustaceana 72: 534–536 Gherardi F, Acquistapace P (2007) Invasive crayfish in Europe: the impact of Procambarus clarkii on the littoral community of a Mediterranean lake. Freshwater Biology 52(7): 1249– 1259, http://dx.doi.org/10.1111/j.1365-2427.2007.01760.x Gherardi F, Barbaresi S (2007) Feeding preferences of the invading crayfish, Procambarus clarkii. Bulletin Français de la Pêche et de la La Pisciculture 385: 7–20, http://dx.doi.org/ 10.1051/kmae:2007014 Graça MAS (2001) The role of invertebrates on leaf litter decomposition in streams – a review. International Review of Hydrobiology 86(4–5): 383–393, http://dx.doi.org/10.1002/15222632(200107)86:4/5<383::AID-IROH383>3.0.CO;2-D Graça MAS, Maltby L, Calow P (1993) Importance of fungi in the diet of Gammarus pulex and Asellus aquaticus: feeding strategies. Oecologia 93(1): 139–144 Gutiérrez-Yurrita PJ, Sancho G, Bravo MÁ, Baltanás Á, Montes C (1998) Diet of the Red Swamp Crayfish Procambarus clarkii in Natural Ecosystems of the Doñana National Park Temporary Fresh-Water Marsh (Spain). Journal of Crustacean Biology 18(1): 120–127, http://dx.doi.org/10.2307/1549526 Hall RO, Meyer JL (1998) The trophic significance of bacteria in a detritus-based stream food web. Ecology 79: 1995–2012, http://dx.doi.org/10.1890/0012-9658(1998)079[1995:TTSOBI]2.0.CO;2 Harris JM (1993) The presence, nature, and role of gut microflora in aquatic invertebrates: a synthesis. Microbial Ecology 25(3): 195–231, http://dx.doi.org/10.1007/BF00171889 Hein CL, Roth BM, Ives AR, Zanden MJV (2006) Fish predation and trapping for rusty crayfish (Orconectes rusticus) control: a whole-lake experiment. Canadian Journal of Fisheries and Aquatic Sciences 63: 383–393, http://dx.doi.org/10.1139/f05-229 Hermanides-Nijhof EJ (1977) Aureobasidium and allied genera. Studies in Mycology 15: 141–176 Hinton MG, Maunder MN (2003) Methods for standardizing CPUE and how to select among them. Proceeding of 16th meeting of the standing committee on Tuna and Billfish. Mooloolaba, Australia, July 9-16, 2003. Inter-American Tropical Tuna Commission La Jolla, California USA, p 11 Huner JV (1988) Procambarus in North America and elsewhere. In: Holdich DM, Lowery RS (eds), Freshwater crayfish: biology, management and exploitation. Croom Heim, London, pp 239–261 Ilhéu M, Acquistapace P, Benvenuto C, Gherardi F (2003) Burrowing of the red swamp crayfish (Procambarus clarkii) in a temporary stream of south Portugal. Archiv für Hydrobiologie 157: 535–546, http://dx.doi.org/10.1127/00039136/2003/0157-0535 Khalil RH, El-Banna SA, Mahfouz NB (2009) Study on micro flora of wild freshwater crayfish (Procambarus clarkii). Proceedings of the 2nd Global Fisheries and Aquaculture Research Conference, pp 257–261 Kimura H, Harada K, Hara K, Tamaki A (2002) Enzymatic approach to fungal association with arthropod guts: a case study for the crustacean host, Nihonotrypaea harmandi, and its foregut fungus, Enteromyces callianassae. Marine Ecology 23(2): 157–183, http://dx.doi.org/10.1046/j.1439-0485.20 02.02778.x Klich MA (2002) Identification of common Aspergillus species. Centraalbureau voor Schimmelcultures, Utrecht, 116 pp Kraus-Epley KE, Moore PA (2002) The effects of antennal lesions on orientation behaviour of the crayfish Orconectes rusticus. Chemical Senses 27: 49–55, http://dx.doi.org/10.1093/ chemse/27.1.49 Lodge DM, Hill AM (1994) Factors governing species composition, population size, and productivity of cool-water crayfishes. Nordic Journal of Freshwater Research 69: 111– 136 Lodge DM, Deines A, Gherardi F, Yeo DCJ, Arcella T, Baldridge AK, Barnes MA, Chadderton WL, Feder JL, Gantz CA, Howard GW, Jerde CL, Peters BW, Peters JA, Sargent LW, Turner CR, Wittmann ME, Zeng Y (2012) Global introductions of crayfishes: evaluating the impact of species invasions on ecosystem services. Annual Review of Ecology and Systematics 43: 449–472 Maraun M, Martens H, Migge S, Theenhaus A, Scheu S (2003) Adding to the enigma of soil animal diversity: fungal feeders and saprophagous soil invertebrates prefer similar food substrates. European Journal of Soil Biology 39: 85–95, http://dx.doi.org/10.1016/S1164-5563(03)00006-2 Mickënienë L, Šyvokienë J (1996) Microflora in the digestive tract of freshwater crayfish. Freshwater Crayfish 11: 441– 449 Minitab Inc., State College, PA, USA (2007) Meet Minitab 15 for windows, USA Momot WT (1995) Redefining the role of crayfish in aquatic ecosystem. Reviews in Fisheries Science 3(1): 33–63, http://dx.doi.org/10.1080/10641269509388566 Momot WT, Gowing H, Jones PD (1978) The dynamics of crayfish and their role in ecosystems. American Midland Naturalist 99: 10–35, http://dx.doi.org/10.2307/2424930 Morpugo M, Aquiloni L, Bertocchi S, Brusconi S, Tricarico E, Gherardi F (2010) Distribuzione dei gamberi d’acqua dolce in Italia. Acta Biologica 87: 125–132 Nelson PE, Toussoun TA, Marasas WFO (1983) Fusarium species: an illustrated manual for identification. The Pennsylvania State University Press, London, 193 pp Picco AM, Angelini P, Ciccarone C, Franceschini A, Ragazzi A, Rodolfi M, Varese GC, Zotti M (2011) Biodiversity of emerging pathogenic and invasive fungi in plants, animals and humans in Italy. Plant Biosystems 145(4): 988–996, http://dx.doi.org/10.1080/11263504.2011.633118 Pitt JI (1979) The genus Penicillium and its teleomorphic state Eupenicillium and Talaromyces. Academic Press, London, 634 pp Richards LJ, Schnute JT (1986) An experimental and statistical approach to the question: is CPUE an index of abundance? Canadian Journal of Fisheries and Aquatic Sciences 43(6): 1214–1227, http://dx.doi.org/10.1139/f86-151 57 L. Garzoli et al.C. Rodriguez CF, Becares E, Fernandez-Alaez M, Fernandez-Alaez C (2005) Loss of diversity and degradation of wetlands as a result of introducing exotic crayfish. Biological Invasions 7: 75–85, http://dx.doi.org/10.1007/s10530-004-9636-7 Rossi L (1983) Interactions between Invertebrates and Microfungi in Freshwater. Oikos 44(1): 175–184, http://dx.doi.org/10.2307/ 3544059 Samson RA (1974) Paecylomyces and some allied hyphomycetes. Studies in Mycology 6: 1–119 Samson RA, Hoekstra ES, Frisvad JC (2004) Introduction to food- and airborne fungi. Centraalbureau voor Schimmelcultures, Netherlands, 389 pp Savini D (2007) Rinvenimento della specie aliena invasiva Procambarus clarkii (Atracidea: Cambaridae) nella riserva naturale “Bosco Siro Negri” (Zerbolò- Pavia). Acta Biologica 83: 33–37 Savini D, Occhipinti-Ambrogi A (2008) Bad Moon Rising: the Lousiana Swamp Red Crayfish, a threat to the freshwater ecosystems in Lombardy. Memorie Società Italiana di Scienze Naturali, Museo Civico di Storia Naturale di Milano 36: 19–20 Savini D, Occhipinti-Ambrogi A, Nicolao J, Perrone M, Garzoli L, Rodolfi M, Picco AM (2008) Il gambero invasivo Procambarus clarkii (Girard, 1852) nella lanca della riserva naturale integrale “Bosco Siro Negri”. Archivio Geobotanico 11(1–2): 49–58 Savini D, Occhipinti-Ambrogi A, Marchini A, Tricarico E, Gherardi F, Olenin S, Gollasch S (2010) The top 27 alien animal species intentionally introduced by European aquaculture and related activities: stocking, sport fishery and ornamental purposes. Journal of Applied Ichthyology 26: 1–7, http://dx.doi.org/10.1111/j.1439-0426.2010.01503.x 58 Scalici M, Gherardi F (2007) Structure and dynamics of an invasive population of the red swamp crayfish (Procambarus clarkii) in a Mediterranean wetland. Hydrobiologia 583: 309–319, http://dx.doi.org/10.1007/s10750-007-0615-8 Scalici M, Pitzalis M, Gibertini G (2009) Crayfish distribution updating in central Italy. Knowledge and Management Aquatic Ecosystem 394–395(6): 1–8, http://dx.doi.org/10.1051/ kmae/2009017 Sinsabaugh RL, Linkins AE, Benfield EF (1985) Cellulose digestion and assimilation by three leaf-shredding aquatic insects. Ecology 66(5): 1464–1471, http://dx.doi.org/10.2307/193 8009 Souty-Grosset C, Holdich DM, Noël PY, Reynolds JD, Haffner P (2006) Atlas of Crayfish in Europe. Publications Scientifiques du MNHN, Paris, 187 pp Sutton BC (1980) The Coelomycetes. Commonwealth Mycological Institute, Kew, Surrey, England, 696 pp Tricarico E, Vilizzi L, Gherardi F, Copp GH (2010) Calibration of FI-ISK, an invasiveness screening tool for nonnative freshwater invertebrates. Risk Analysis 30(2): 285–292, http://dx.doi.org/10.1111/j.1539-6924.2009.01255.x Türkkan M, Andolfi A, Zonno M, Erper I, Perrone C, Cimmino A, Vurro M, Evidente A (2011) Phytotoxins produced by Pestalotiopsis guepinii, the causal agent of hazelnut twig blight. Phytopathology Mediterranean 50: 154–158 Vasconcelos V, Oliveira S, Teles FO (2001) Impact of a toxic and a non-toxic strain of Microcystis aeruginosa on the crayfish Procambarus clarkii. Toxicon 39: 1461–1470, http://dx.doi.org/ 10.1016/S0041-0101(01)00105-2 Yokoe Y, Yasumasu I (1964) The distribution of cellulase in invertebrates. Comparative Biochemistry and Physiology 13: 323–338, http://dx.doi.org/10.1016/0010-406X(64)90027-1