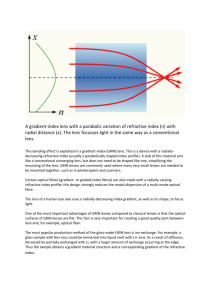
Wide-field schematic eye models with gradient
advertisement

A. V. Goncharov and C. Dainty Vol. 24, No. 8 / August 2007 / J. Opt. Soc. Am. A 2157 Wide-field schematic eye models with gradient-index lens Alexander V. Goncharov* and Chris Dainty Applied Optics Group, Department of Experimental Physics, National University of Ireland, Galway, Ireland *Corresponding author: alexander.goncharov@nuigalway.ie. Received October 20, 2006; revised February 18, 2007; accepted March 18, 2007; posted April 2, 2007 (Doc. ID 76289); published July 11, 2007 We propose a wide-field schematic eye model, which provides a more realistic description of the optical system of the eye in relation to its anatomical structure. The wide-field model incorporates a gradient-index (GRIN) lens, which enables it to fulfill properties of two well-known schematic eye models, namely, Navarro’s model for off-axis aberrations and Thibos’s chromatic on-axis model (the Indiana eye). These two models are based on extensive experimental data, which makes the derived wide-field eye model also consistent with that data. A mathematical method to construct a GRIN lens with its iso-indicial contours following the optical surfaces of given asphericity is presented. The efficiency of the method is demonstrated with three variants related to different age groups. The role of the GRIN structure in relation to the lens paradox is analyzed. The wide-field model with a GRIN lens can be used as a starting design for the eye inverse problem, i.e., reconstructing the optical structure of the eye from off-axis wavefront measurements. Anatomically more accurate age-dependent optical models of the eye could ultimately help an optical designer to improve wide-field retinal imaging. © 2007 Optical Society of America OCIS codes: 330.4060, 110.2760, 330.4460, 080.3620, 010.1080. 1. INTRODUCTION The earliest schematic eye model involving a high refractive index core and a lower index cortex for crystalline lens was proposed nearly a century ago by Gullstrand [1]. This model with five spherical surfaces, usually referred to as Gullstrand’s “No. 1” model, was intended to describe aberrational properties of the human eye on axis. After being revised by Le Grand and El Hage [2] (using a homogeneous index lens), this model has been widely used as a first-order approximation in spite of poor agreement with the measured values of the ocular aberrations. To make theoretical eye models more consistent with experimental data, aspheric surfaces and a lens with a varying refractive index have been considered. In these schematic models, the lens is approximated either by a finite number of concentric “shells” with a slightly different index of refraction or by gradient-refractive-index, or “gradientindex” (GRIN) elements with a smooth index decrease from the lens center to its periphery. In the first group, the index of the lens changes stepwise. For example, in the theoretical eye model proposed by Lotmar [3], the lens was constructed of seven shells with refractive indices varying from 1.38 to 1.41 in steps of 0.005 (at wavelength = 0.543 nm). Pomerantzeff et al. [4] constructed a shell– lens consisting of 398 layers with different indices, radii of curvature, and thickness varying as a function of a high-order polynomial. Al-Ahdali and El-Messiery [5] proposed an eye model incorporating 300 spherical shells in the lens. In the eye model proposed by Popiolek-Masajada [6], the anterior and posterior surfaces of the shell–lens were made hyperboloidal with a rather high value of asphericity compared with that of recent population studies. 1084-7529/07/082157-18/$15.00 Liu et al. [7] established an anatomically more accurate eye model with 602 concentric ellipsoidal shells. Unfortunately, even for such a large number of shells, the noncontinuous structure of the lens produces multiple foci [6]; i.e., the longitudinal spherical aberration (SA) becomes a discontinuous function when the ray enters the lens at certain critical heights. The second group of lens models is free of this effect, since the GRIN lens has a continuous index gradient usually described by a set of equations. Well-known schematic eye models with a GRIN lens are those of Gullstrand [1], Blaker [8], Smith et al. [9], and Liou and Brennan [10]. A good review on the optical properties of the crystalline lens and their significance to image formation was given by Smith [11]. The two kinds of crystalline lens models featuring the continuous GRIN and shell structures are well described in [12]. Both groups of these eye models are applicable only to describing foveal vision (on visual axis), except for Lotmar’s [3] and Pomerantzeff ’s [4] models with a shell lens, which are of high complexity owing to a large number of parameters involved compared with a GRIN lens model. Therefore, we believe there is a need for a simpler wideangle model with a GRIN lens to describe aberrational properties of the eye at oblique angles, which is of great importance for peripheral vision and imaging of the peripheral fundus. Together with Lotmar’s and Pomerantzeff ’s models, there have been several attempts to develop wide-field models using a lens of constant refractive index. The Gullstrand–Le Grand model [2] was modified by Kooijman [13], who introduced a moderate asphericity 共k = −0.25兲 on both surfaces of the cornea and a relatively © 2007 Optical Society of America 2158 J. Opt. Soc. Am. A / Vol. 24, No. 8 / August 2007 large asphericity on the anterior and posterior surfaces of the homogenous lens (k = −3.06 and k = −1, respectively) to obtain a wide-angle model. Shortly after, Navarro et al. [14] proposed a schematic eye model with similar asphericities of the corneal surfaces (k = −0.26 and 0) and surfaces of the lens 共k = −3.1316 and −1.0) derived from averaged anatomical measurements obtained in vitro by Howcroft and Parker [15]. Navarro’s eye model had a lens with a homogenous index changing with accommodation. The off-axis aberrations of the unaccommodated form of this model was extensively analyzed later by EscuderoSanz and Navarro [16]. Navarro’s model agrees well with experimental findings for off-axis aberrational properties of the real eye, yet it does not model the graded-index structure in the crystalline lens. Therefore it would be very appropriate to include such anatomical features of the lens into the eye model, especially if one needs to use it for solving the eye inverse problem, i.e., reconstructing the optical structure of the eye from off-axis wavefront measurements. In the present paper, a simplified wide-field eye model with a GRIN lens, to be used as a starting point for solving the eye inverse problem, is proposed and is optimized to fit both chromatic aberrations and overall root-meansquare (RMS) wavefront error observed at different visual field angles. The experimental data for chromatic aberrations is taken from a simple chromatic eye model developed by Thibos and colleagues [17]. This model being valid on axis, after a minor modification of the refractive surface shape, its asphericity also provides the correct amount of SA [18]. We shall refer to this modified model as the Indiana eye, which we use on axis, since Navarro’s model shows a slightly excessive amount of SA. The experimental data for RMS wavefront error as a function of visual field angle is taken from averaged measurements of two different studies by Navarro et al. [19] and Atchison and Scott [20,21] carried out with a laser ray-tracing technique [22,23] and the Shack–Hartmann (SH) wavefront sensor [24–26], respectively. These studies seem to indicate a similar amount of off-axis aberrations of the order of those predicted by Navarro’s schematic eye, which suggests that Navarro’s model is a good starting point for deriving a new wide-field mode of the eye with a GRIN lens. Recent advances in the understanding of the refractive index distribution [27] in the crystalline lens make it possible to narrow down our search for promising solutions to fit simultaneously the aberrations of the Indiana eye on axis and Navarro’s model off axis in one single wide-field model with a GRIN lens matching the typical distribution of the refractive index. The ultimate goal is to find a relatively simple function of a few physical parameters representing the index distribution within the lens, which can be easily adjusted while reconstructing the structure of the real eye from off-axis wavefront measurements. The success of such a reconstruction will depend on the initial schematic eye model used and the correctness of the index distribution in the lens. Therefore it is important to develop a new eye model closely resembling the anatomical structure of the real eye and providing just a few efficient parameters to account for intersubject variability and age effects in the GRIN lens [28]. A. V. Goncharov and C. Dainty 2. ANALYTICAL MODEL OF THE GRIN LENS Ideally, a new schematic eye model should predict accurately the aberrations arising at each ocular component as well as the overall ocular aberrations. As pointed out by Smith [11], even using current sparse data on the optical structure of the lens, one could use mathematical modeling to study the effects of surface shapes and gradient refractive index structure on the Gaussian and aberration properties of the lens. We believe that using a GRIN lens in the schematic eye could facilitate our search for a more accurate model of the optical system matching the experimental data for on-axis and off-axis measurements of ocular aberrations. In our case, the averaged experimental data are implicitly presented in the Indiana and Navarro eye models. The GRIN lens should have a realistic anatomical structure of the crystalline lens consistent with the characteristic distribution of the refractive index reported by many researchers [27,29]. As we mentioned in the introduction, there have been several attempts to employ a GRIN lens for modeling of ocular aberrations on axis (at one field point) [1,8–10,30], but to our knowledge off-axis aberrations have been modeled only with a homogeneous index lens [13,14,16] or a shell– lens [3,4]. Interestingly, wide-angle eye models of a rainbow trout [31] and octopus [32] have been constructed using a GRIN lens and analyzed for off-axis performance. These studies suggest that a spherical symmetry of the GRIN lens allows maintenance of a well-corrected retinal image far into the peripheral field. Both lens models have a strong refractive index gradient over nearly the same range, increasing from 1.38 at the lens cortex to 1.50 at the center. To describe this index gradient, a polynomial of the tenth order (as a function of radial distance from the lens center) has been used. The human crystalline lens does not have spherical symmetry, yet it is assumed to be rotationally symmetrical about its optical axis. Early studies of the refractive index distribution in the human lens by Nakao et al. [33] using an interference technique showed that the index profile, in both sagittal and equatorial sections, could be approximated by an even polynomial of second order. However, experimental studies by Pierscionek et al. [34,35], based on an equatorial-to-sagittal transposition method developed by Chan et al. [36], showed that the distribution of refractive index in the human lens has a nonparabolic profile; it is relatively flat over the inner two thirds of the lens with a steep falloff in the cortical region. A similar type of distribution was found along the optical axis of the human lens for protein concentration [37]. More recent studies by Peirscionek [29,38] using a reflectometric fiber optic sensor [39] for direct measurements of refractive index along the equatorial and sagittal planes showed nearly flat index profile in the aged lenses (for both planes), whereas young lenses exhibited a rapid decrease of refractive index at the periphery in the equatorial plane and relatively low gradient in the sagittal plane. The latest studies by Moffat et al. [40] and Jones et al. [27] using a noninvasive magnetic resonance imaging technique obtained refractive index maps through crystalline lenses. It was confirmed that with increasing age, index profiles become flatter in the central region and steeper at the periphery, A. V. Goncharov and C. Dainty Vol. 24, No. 8 / August 2007 / J. Opt. Soc. Am. A especially in the equatorial plane. This fact explains the earlier difficulty in fitting the equatorial index profile to a second-order polynomial. Therefore we consider modeling the lens structure of young adults only, leaving a more complex case of irregular, nonsymmetric lenses with a flat central index profile for future investigation. A. General Model of the GRIN Lens In our analytical model for a rotationally symmetric GRIN lens of axial thickness d, we assume that it consists of two parts. For the first, anterior part of the lens, the refractive index n is described by a fourth-order even polynomial of radial distance r from the optical axis in the equatorial plane and by a fourth-order polynomial of longitudinal distance z measured from the lens anterior surface in the sagittal plane: na共z,r兲 = n0 + n1r2 + n2r4 + n3z + n4z2 + n5z3 + n6z4 , 共1兲 where n0 is the refractive index at the anterior surface of the lens and n1, n2, n3, n4, n5, and n6 are GRIN lens coefficients. The refractive index distribution along the optical axis na共z兲 is an increasing function ranging from n0 to nmax. For the second, posterior part of the lens, the refractive index is also described by a fourth-order polynomial: np共z,r兲 = nmax + n1r2 + n2r4 + n3,2z + n4,2z2 + n5,2z3 + n6,2z4 , 共2兲 where nmax is the refractive index at the intermediate plane, where the refractive index reaches its maximum value, and n1, n2, n3,2, n4,2, n5,2, and n6,2 are GRIN lens coefficients. Here the longitudinal distance z is calculated from the plane of maximum refractive index (peak plane) so that the axial distribution np 共z兲 is a decreasing function ranging from nmax to n0. Our task is to find such a refractive index distribution within the GRIN lens, which corresponds to the right amount of SA and at the same time has an anatomically sound structure; that is, iso-indicial lines of constant refractive index should follow the optical surfaces of the lens. The latter condition can be fulfilled in the rotationally symmetric GRIN lens of the form given by Eqs. (1) and (2) if the optical surfaces of the lens are conicoids (a conicoid is formed by rotating a conic section about its axis of symmetry). To derive formulas for the GRIN lens coefficients, we assume that the anterior lens surface has a radius of curvature ra and conic constant ka; therefore the iso-indicial contour at the anterior vertex of the lens has to follow the shape of the lens surface, given by the conic section equation r2 = 2raz − 共1 + ka兲z2 . 共3兲 Using Eq. (3) for r2 in Eq. (1) and regrouping the sum as a fourth-order polynomial of z, we obtain four linear relationships for GRIN lens coefficients by equating to zero the coefficients of the polynomial. For our derivation, we denote by zm the distance from the anterior surface to the peak plane. To reach the maximum value nmax in this plane, one has to fulfill two conditions: na共zm兲 = nmax and na⬘ 共zm兲 = 0, where na⬘ 共zm兲 is the derivative of na with re- 2159 spect to z evaluated at z = zm. Similarly, for the posterior lens surface we have r2 = 2rpt − 共1 + kp兲t2 , 共4兲 where rp and kp are the radius of curvature and conic constant of the surface, respectively, and t is a parameter defining the axial coordinate at the posterior vertex of the lens such that 共5兲 z = t + d − zm . Using Eqs. (4) and (5) in Eq. (2) and regrouping the sum as a fourth-order polynomial of t, we obtain four additional relations for GRIN lens coefficients by equating to zero the coefficients of the polynomial. For the second part of the lens, the condition for reaching the maximum refractive index in the peak plane becomes simply n3,2 = 0. The derivation of the formulas for GRIN lens coefficients from these relations is straightforward; therefore we present here only the final expressions. Introducing two auxiliary parameters, ⌬n = 共nmax − n0兲, 2 m = zm 共1 + ka兲 − 2razm , the index coefficients can be expressed in the following forms: n1 = 2⌬n/m, n2 = ⌬n/m2, n3 = − 4⌬nra/m, n4 = − 2⌬n关3ra2 − 共ra − zm共1 + ka兲兲2兴/m2 , n5 = 4⌬n共1 + ka兲ra/m2, n6 = − ⌬n共1 + ka兲2/m2 , n3,2 = 0, n5,2 = 4⌬n共1 + kp兲关rp + 共d − zm兲共1 + kp兲兴/m2 , n6,2 = − ⌬n共1 + kp兲2/m2 , n4,2 = 共1 + kp兲n1 − 4n2rp2 − 3共d − zm兲n5,2 − 6共d − zm兲2n6,2 . 共6兲 The location of the peak plane in the GRIN lens is found from a quadratic equation 2 共kp − ka兲zm − 2关d共1 + kp兲 + rp − ra兴zm + d关d共1 + kp兲 + 2rp兴 = 0. 共7兲 It is worth noticing that for a given aspheric shape of the lens (defined by its geometrical parameters ra, rp, ka, kp, and d) and chosen optical parameter ⌬n, the refractive index distribution in the GRIN lens is uniquely defined by Eqs. (6) and (7). The refractive index at the anterior and posterior surfaces of the lens is equal to n0. This represents the case of a balanced GRIN lens; that is, all isoindicial contours of the lens complete each other on both sides of the peak plane. We should also mention one interesting feature of this model. If kp + 1 ⬎ −rp / d, then there exists an additional solution for choosing the peak plane location zm, 2160 J. Opt. Soc. Am. A / Vol. 24, No. 8 / August 2007 A. V. Goncharov and C. Dainty zm = d + rp/共1 + kp兲. 共8兲 In this case, the refractive index np共d − zm , 0兲 at the posterior lens surface is higher than the anterior surface refractive index n0, and thus we shall call this case an unbalanced lens. Note that inserting zm from Eq. (8) into Eq. (6) for the expression of n5,2 leads to n5,2 = 0 and that from Eq. (2) we find np共d − zm , 0兲 = nmax + n4,2共d − zm兲2 + n6,2共d − z m兲 4. B. Simplified Model of the GRIN Lens It is possible to simplify the model of the GRIN lens if we relax the condition of iso-indicial contours being coincident with the external surfaces of the lens. In such a case, the refractive index distribution in the GRIN lens is described by Eq. (1) as a single element with index coefficients defined by three parameters of the lens shape ra, rp, and d; index range ⌬n; and two free parameters zm and n2, which are used to set the refractive index n0 and amount of SA in the lens within the expected range, respectively. Similar to the previous derivation of a balanced GRIN lens, we find the index coefficients in the following explicit forms: n1 = − ⌬nzmd 共d − 2zm兲共d − zm兲/m , 2 * * 2 n4 = − ⌬nd关d3ra − 3d共3ra + rp兲zm 3 + 4共2ra + rp兲zm 兴/m* , n5 = 2⌬n关d3ra − d2共3ra + rp兲zm 2 + 3共ra + rp兲zm 兴/m* , 共9兲 2 2 共d − zm兲2关ra共d − zm兲2 + rpzm 兴. This simplified where m* = zm model of the GRIN lens has its iso-indicial contours coincident with the optical surfaces only near their vertices, given that the asphericity of the surfaces was excluded from the parameters of the lens shape. The index coefficient n2 and location of the peak plane zm are responsible for the aspheric shape of the marginal iso-indicial contours, which have a refractive index of n0 and pass through the vertices of the lens. In spite of the highly aspheric shape of the marginal contours, which cannot be described as a conicoid, we can approximate their equivalent conic constant by using a second term in the Taylor series expansion. The asphericities of anterior and posterior marginal iso-indicial contours are − zm兲共d − 2zm兲兴, kb = − 1 + 关n4 + dn5 − 4rp共n2rp − n1/d兲兴m*/关⌬nzmd2 ⫻共d − zm兲共d − 2zm兲兴. F = − 6n0n1d/共3n0 − 2n1d2兲. 共11兲 It can be seen that the lens power depends on refractive index n0 and coefficient n1, which is a function of zm; therefore we may adjust the power of the lens and the whole eye by simply choosing the appropriate position of the peak plane while retaining the value of n0 at the expected level, which is about 1.37 according to a recent study by Jones and colleagues [27]. Following Smith and Atchison [42], we calculate the primary SA coefficient W4,0 of the simplified GRIN lens using the Seidel aberration coefficient SI, which corresponds to third-order SA. These coefficients are related as follows: W4,0 = SI/共8h04兲. 共12兲 where the individual contributions are related to the GRIN lens coefficients by the equations n6 = − ⌬n关d2ra − 2d共2ra + rp兲zm ka = − 1 − 共n4 + where g = n1 / 共n0 − 2n1d2 / 3兲 for our GRIN lens models. Assuming the entrance height h0 = 1, the equivalent power of the GRIN lens bulk can be expressed as follows: W4,0 = Wa + Wb1 + Wb2 + Wp , 3 + 2共ra + rp兲zm 兴/m* , 4n2ra2兲m*/关⌬nzmd2共d h共z兲 = h0共1 + gz2兲, For the eye, the wavefront aberration is usually of the order of 10−3 mm; hence it is more practical to use the units of the primary SA in micrometers, while the ray height is expressed in millimeters. With this in mind, we derive an expression for the primary SA of the GRIN lens as a sum of the anterior surface refractive contribution Wa, two transfer contributions of the GRIN lens bulk Wb1 and Wb2, and the posterior surface refractive contribution Wp: n3 = 2⌬nzmrad 共d − 2zm兲共d − zm兲/m , 2 that is, the height of the ray h 共z兲 above the optical axis is given by 共10兲 The equivalent optical power of the GRIN lens can be approximately calculated assuming a parabolic ray path through the lens as suggested by Smith and Atchison [41]; Wa = 500共n1 + n3/4ra兲/ra , Wb1 = 25F4共− 6300n02 + 588n0n1d2 + n1d3共1260n3 + 1080n4d + 945n5d2 + 840n6d3 − 184n1d兲兲/1512n04n1d, Wb2 = 25F4n2共3780n03n1d2 − 3402n02n12d4 + 1236n0n13d6 − 187n14d8 − 2835n04兲/1134n04n14d3 , Wp = − 500关n1 + 共n3 + 2n4d + 3n5d2 + 4n6d3兲/4rp兴/rp . 共13兲 These equations are derived from a basic expression of Sands [43], who established the formula for third-order SA (Seidel sum SI) of a GRIN medium. Since we assumed again a parabolic ray path through the medium, Eq. (12) is an approximation. It can be seen that the second transfer contribution Wb2 depends on the index coefficient n2, and as we show later it plays a major role in balancing the amount of SA in the GRIN lens. A. V. Goncharov and C. Dainty Vol. 24, No. 8 / August 2007 / J. Opt. Soc. Am. A Modeling older eyes with a steeper refractive index profile in the equatorial plane might require polynomials of higher order of r in Eqs. (1) and (2); for example, the University of Rochester representation includes two extra terms r6 and r8, a modified version of which has been used for modeling relaxed and accommodated states of the crystalline lens [44]. Introducing these additional terms of even power of the radial coordinate r will affect the amount of high-order SA of the lens, but not its optical power. 3. MODELING THE SPHERICAL ABERRATION OF THE EYE The SA of the eye has been extensively studied by many researchers [45–47], yet its relative contribution to ocular 2161 aberrations is a subject of debate. For comparison, we have collected from the literature the experimental data on ocular SA in Table 1. Various psychophysical methods (PM) for direct measurements of the longitudinal SA (LSA) show a relatively large amount of aberration [45–47] except for a study by Millodot and Sivak [48]. The latter work was chosen as the basis for the linear model of LSA proposed by Liou and Brennan [49], for which the value of LSA was converted into dioptric power changes ⌬F = n/共f⬘ + LSA兲 − n/f⬘ , where f⬘ is the equivalent focal length of the eye in meters and n is the refractive index of the last refractive medium, leading to a linear relationship between LSA and ray height h (in millimeters) at the entrance pupil of the eye: ⌬F = 0.2 h. This LSA linear model was used by Liou Table 1. Summary of Experimental Data for the Ocular and Corneal Spherical Aberrations of the Human Eye and Different Eye Modelsa Ocular SA Corneal SA Number of Eyes Mean Age (years) ⌬F (D) W40 共m / mm−4兲 Z40 共m兲 W40 Z40 Pupil Diameter 20 10 12 3 218 200 13 13 2 15 15 7 10 75 90 228 72 30 5 27 16 16 — — — 33 42 26 35 66 39 25 68 35 60 44 18 50 45 21 30 25 45 65 0.5 0.4 0.7 1.5 — — — — 0.8 — — — — — — — — — — — — — — — — — — — 0.005 0.013 — — — — — — — — — — 0.014 — — — — — — — 0.138 0.120 — — — 0.095 0.175 0.110 0.303 0.175 0.060 — 0.160 0.132 — — — — — — — — — — 0.032 0.029 — — — — — — — — — — 0.074 — — — — — — — — — — — — — — — — 0.255 0.300 0.281 0.260 0.207 — 0.192 0.260 0.265 6.0 4.0 6.0 6.2 5.7 6.0 5.4 5.4 5.6 6.0 6.0 5.9 5.9 6.0 6.3 6.0 6.0 6.0 4.0 6.0 6.0 6.0 Eye models SA1, Liou and Brennan [10] SA2, Indiana eye [18,50] Navarro [14,16] 45 — — 0.6 1.5 1.7 0.016 0.031 0.033 0.095 0.187 0.199 0.035 — 0.027 0.211 — 0.166 6.0 6.0 6.0 20U 20B 20S 20 20 20 0.7 0.6 0.6 0.013 0.012 0.013 0.078 0.071 0.075 0.040 0.040 0.040 0.241 0.241 0.241 6.0 6.0 6.0 30U 30B 30S 30 30 30 1.3 1.1 1.2 0.021 0.020 0.022 0.128 0.121 0.132 0.043 0.043 0.043 0.260 0.260 0.260 6.0 6.0 6.0 40U 40B 40S 40 40 40 1.5 1.3 1.4 0.029 0.027 0.030 0.174 0.162 0.179 0.047 0.047 0.047 0.283 0.283 0.283 6.0 6.0 6.0 Study/Method Millodot and Sivak [48]/PM Ivanoff [46]/PM Jenkins [47]/PM Koomen et al. [45]/PM Porter et al. [52]/SH Thibos et al. [53]/SH Smith et al. [55]/CA, VK Salmon et al. [26]/SH, PM Calver et al. [56]/CA Artal et al. [57]/SH Amano et al. [58]/SH He et al. [54]/PM, VK Wang et al. [70]/VK Alió et al. [59]/SH, VK Kelly et al. [71]/SH, VK Artal and Guirao [68]/SH, VK Guirao et al. [62]/VK a PM, psychophysical methods; SH, Shack–Hartmann; CA, crossed-cylinder aberroscope; VK, videokeratographic system; U, unbalanced; B, balanced; S, simplified. 2162 J. Opt. Soc. Am. A / Vol. 24, No. 8 / August 2007 and Brennan in their subsequent finite model of the eye with a GRIN lens [10]. As pointed out by Thibos et al. [50], before using raw psychophysical data to estimate the amount of ocular SA, one has to account for odd aberrations, such as coma, which arise on axis due to the lack of rotational symmetry in real eyes. They illustrated the concept for eliminating the coma contribution from measurements of transverse aberration of the eye [51]. The estimated amount of the SA was modeled by a reduced schematic eye with an elliptical refracting surface [50], for which k = −0.43. The latter model was consistent with their earlier single-surface chromatic eye model [18]. In that respect only psychophysical measurements by Koomen et al. [45], utilizing an annular pupil mask with different diameters, provided averaging of the coma contribution, and their results are in good agreement with the Indiana eye model. On the other hand, wavefront measurements with modern wavefront sensing techniques [24] do not always agree with the Indiana eye model. Several studies [52,53] of ocular wavefront aberrations in large populations using the SH sensor showed that in a fourth-order Zernike expansion the mean value of coefficient Z40, responsible for primary SA, is significantly different from zero. In a large study by Porter et al. [52], the mean value of Z40 was found to be somewhere in between the value predicted by the Indiana eye model and that of Liou and Brennan listed in Table 1. However, two large studies of young subjects by Thibos et al. [53] and He et al. [54] clearly supported Liou and Brennan’s model. Similarly, analysis of the data obtained by Smith et al. [55] using a crossed-cylinder aberroscope (CA) to measure the mean value of the primary SA coefficient W4,0 supports Liou and Brennan’s model. It is worth mentioning that for a given pupil size, one can use a simple relation W4,0 = 6冑5Z40 , provided that there is no defocus error in the eye. Another recent study [26] comparing a psychophysical method with the SH wavefront sensor demonstrated consistency of both techniques on two subjects. The estimated amount of the LSA in dioptric power was somewhere in the middle of these two models. Due to a relatively small number of measurements currently available for ocular SA, and in view of its large intersubject variability, it is reasonable to keep both options for representing ocular SA: Liou and Brennan’s model [10], hereafter simply called the SA1 case, and the Indiana eye model [18,50] with a single ellipsoidal refracting surface 共kSA2 = −0.43兲, referred to as the SA2 case. The SA of the SA1 case can be accurately reproduced by the Indiana eye model with a modified ellipsoidal surface 共kSA1 = −0.495兲. Therefore we shall use only the Indiana eye model with an appropriate conic constant k to mimic both SA1 and SA2 cases. Figure 1 depicts the ocular SA for four cases, including Navarro’s wide-angle eye model. A comparative analysis of the wavefront measurements of the Zernike coefficient Z40 for primary SA sorted into two different age groups of young adults and middle-aged people as given by Porter et al. [52] and Calver et al. [56] indicates that positive value of Z40 increases with age, A. V. Goncharov and C. Dainty which is consistent with findings of other studies by Smith et al. [55], Artal et al. [57], Amano et al. [58], and Alió et al. [59]. In spite of intersubject variability, we can deduce from these studies that the primary SA of young adults seems to favor the SA1 case, whereas the SA2 case is more appropriate for middle-aged people. 4. EFFECT OF AGING ON THE ANATOMICAL STRUCTURE OF THE EYE Constructing a new schematic eye model that is structurally similar to the human eye demands a thorough consideration of the biometric data. Empirical values of ocular parameters available in the literature display a mixed effect of intersubject variability and restructuring of the eye due to aging. Averaging such diverse biometric data without taking into account the effect of aging on anatomical structure is more likely to result in some unrealistic eye model, which might not be applicable even for a specific age group. Therefore, we consider here three age groups and their corresponding schematic models representing 20-, 30-, and 40-year-old eyes. Since averaging biometric data specifically for each group is not always possible, we used data from various experimental studies describing the changes in shape and internal geometry of the human eye as a function of age. The resulting geometrical parameters of our three models are listed in Table 2, with references to the original data given below. The optical layout of the eye is shown on the right-hand side in Fig. 2. A. Anterior Cornea According to the data analysis by Lam and Douthwaite [60], the correlation between the cornea anterior radius rca and posterior radius rcp derived from a regression line for horizontal meridian gives rcp = 0.87rca − 0.24. On the other hand, estimating the average ratio rcp / rcp for our age groups gives the mean value of 0.84, which is comparable to Edmund’s study [61]. In vertical meridian, the cornea shape is less curved and the average ratio rcp / rcp is about 0.83. Since data are available only for offaxis aberration in the eye measured in the horizontal field [19–21], we have adopted here the averaged values for the corneal anterior radius of curvature in horizontal meridian from the data of Lam and Douthwaite [60] and more recent data by Guirao et al. [62] derived from measurements with a videokeratographic system (VK) [63]. We also use Guirao’s data to account for the effect of aging on the conic constant kca of the anterior cornea surface, which becomes less prolate with increasing age. Our chosen values for kca in Table 2 agree well with findings by Sheridan et al. [64] 共kca = −0.11兲 and Aoshima et al. [65] 共kca = −0.08兲 but are slightly higher than the mean value of− 0.18± 0.18 reported in other studies [66,67]. We are aware of this discrepancy and are ready to support our choice by additional analysis of experimental data for corneal SA converted for a 6 mm pupil. Table 1 contains SA coefficients W40 and Z40 of the anterior corneal surface estimated for three age groups (20, 30, and 40 years) with the parameters rca and kca given in Table 2. We assumed A. V. Goncharov and C. Dainty Vol. 24, No. 8 / August 2007 / J. Opt. Soc. Am. A 2163 Fig. 1. (Color online) Longitudinal spherical aberration of the eye predicted by different models: 0, linear model based on Millodot and Sivak’s data [48]; 1, Liou and Brennan’s eye model with a GRIN lens [10]; 2, Indiana eye model with a single ellipsoidal refracting surface [50] 共k = −0.43兲; 3, Navarro’s wide-angle eye model [16]; 20U, 30U, and 40U models with an unbalanced GRIN lens; 20B, 30B, and 40B models with a balanced GRIN lens; 20S, 30S, and 40S models with a simplified GRIN lens. obtained by Amano et al. [58] gave Z40 = 0.26 m. These two large studies showed no statistically significant correlation between age and corneal SA. On the other hand, in a more recent study by Alió et al. [59], the corneal SA Zernike sum 共Z40 + Z60兲 showed a weak increase with age, starting at 0.26 m (for 20-year age) and reaching 0.28 m at the age of 40 years. For comparison, we that the corneal refractive index is 1.375 at = 589 nm, which is our reference wavelength. Artal and Guirao [68] estimated Seidel aberration coefficients; the mean value was W40 = 0.04 m / mm4. Two studies of young subjects by He et al. [54,69] showed the mean value Z40 = 0.3 m. In the large study by Wang et al. [70], the mean value was Z40 = 0.28 m, whereas the data Table 2. Effect of Aging on the Anatomical Structure of the Human Eye Average Model Age (Years) Anatomical Structure Cornea anterior radius, rca (horizontal) Cornea posterior radius, rcp (horizontal) Cornea anterior conic constant, kca Cornea posterior conic constant, kcp Central cornea thickness Anterior chamber depth (ACD) Lens thickness, d Lens anterior radius, ra Lens posterior radius, rp Vitreous chamber depth (VCD) 20 30 40 7.85 6.59 −0.12 −0.23 0.55 3.28 3.49 12.28 −7.87 16.58 7.76 6.52 −0.10 −0.30 0.55 3.06 3.69 11.51 −7.67 16.60 7.67 6.44 −0.08 −0.37 0.55 2.84 3.89 10.74 −7.47 16.62 2164 J. Opt. Soc. Am. A / Vol. 24, No. 8 / August 2007 A. V. Goncharov and C. Dainty ridional profile of the posterior corneal surface was approximated by an eighth-order aspheric, which after refitting to a conicoid (1 m fitting error) gave us an average kpc = −0.22. Therefore we chose the conic constant kpc to be in the range of these two studies. The choice of this parameter is not critical, because the contributions to ocular SA from the posterior corneal surface is not significant due to the small difference between corneal and aqueous refractive indices, the latter being 1.3374 as in Navarro’s eye model at = 589 nm. Fig. 2. (Color online) Optimization of the eye model by reverse ray tracing. For clarity, optical systems are shown on both sides of the reference plane (vertical dashed line), where rays start to traverse backward. should also give typical values of SA for eyes with more negative values of kca. Assuming corneal radius of curvature ra = 7.85 mm and kca = −0.18, we get less SA from the cornea as Z40 = 0.20 m and W40 = 0.033 m / mm4 due to its gradual decrease to zero at kca = −0.53. The majority of experimental data in Table 1 (except for data by Kelly et al. [71]) indicates the significance of the corneal contribution to the ocular SA, and hence we choose a relatively small absolute value of its conic constant. However, a more recent study by Navarro et al. [72] of the mean shape of the anterior cornea showed a more negative conic constant k ⬍ −0.4, which would predict much lower corneal SA. The reasons for such a striking difference might be due partly to a discovered 2.5 deg tilt of the corneal axis with respect to the optical axis of the lens, whereas other studies assumed rotational symmetry of the eye. It is clear that different authors would tend to choose somewhat different anatomical parameters for their models according to their current knowledge. However, due to rapid evolution in the field, some biometric data may soon become outdated. For this reason we will focus on the methodology of the construction of the GRIN lens for a given set of optical parameters, assuming rotational symmetry of the eye. B. Posterior Cornea In a large study of 500 eyes (500 subjects, mean age = 31 years) by Lleó et al. [73], there was no significant correlation found between central corneal thickness and age. The mean corneal thickness for a young group (283 subjects, range of 18– 30 years) was 0.545 mm, for the second group (155 subjects, range of 31– 40 years) 0.551 mm, and for the third group (62 subjects, range of 41– 67 years) 0.549 mm. A comparable mean value of 0.546 mm was found in a study of corneal thickness for 92 eyes (46 subjects, mean age= 31 years) by Lam and Chan [74]. We chose the average value of 0.55 mm for corneal thickness in all our models. The radius of curvature for the posterior corneal surface is found as rcp = 0.84rca. The asphericity of the posterior corneal surface is known with less confidence; according to Dubbelman et al. [67], the mean value of kpc is −0.38± 0.27 and the surface becomes more prolate (kpc becomes more negative) with increasing age, showing a trend of −0.007 per year, which we adopted in our models. In a study by Aoshima et al. [65], based on corneal topography with the Orbscan II system, the me- C. Anterior Segment and Crystalline Lens The accuracy of estimating the curvature and especially the asphericity of the lens surfaces is limited by the correction technique used for interpreting the Scheimpflug slit images of the eye [75]. The raw images of the lens shape are distorted due to the refractive properties of the cornea and more significantly due to the GRIN structure of the lens itself. To take into account these factors one needs some additional knowledge of the corneal shape and the GRIN structure. The latter aspect presents a real challenge (Dubbelman et al. [76]). This uncertainty creates a large degree of diversity in the literature results obtained from data analysis of the age-related changes in the lens shape. Koretz et al. [77] supported their data analysis with independent magnetic resonance imaging, which we use in our models; however, the eye stability over the scanning period and the finite size of the pixels limit the resolution of this technique, and therefore other correction methods might provide more accurate results (see Dubbelman and Van der Heijde [78]). The geometry of the anterior segment of the human eye as a function of age is based on averaged data from highresolution magnetic resonance images and Scheimpflug slit-lamp images by Koretz et al. [77,79]. For the anterior chamber depth (ACD) and lens thickness d expressed in millimeters, we adopted the following age functions: ACD= 4.27− 0.022A and d = 3.09+ 0.02A, where A is a parameter of age in years. Using these expressions we can estimate dependence of the anterior segment length (ASL) on age: ASL= ACD+ d = 7.36− 0.002A, which is in good agreement with the data obtained with Scheimpflug imaging [79]. The age function for lens thickness is comparable with another study by Alió et al. [59]. We define the radius of curvature for the anterior and posterior surfaces of the crystalline lens as ra = 13.82− 0.077A and rp = −8.27+ 0.02A, respectively; the latter expression is comparable with earlier findings by Brown [80]. We should note that the posterior surface of the lens is one of the most challenging objects to characterize because its location is the least accessible for imaging; as a consequence, the age function chosen for rp may contain larger uncertainty than other parameters of the eye. Ideally, for a constant length of the globe (axial distance between cornea and retina), assuming its average value of 23.9 mm from two large studies [81,82], the vitreous chamber depth (VCD) has to slightly increase with age: VCD= 16.54+ 0.002A, which is reflected in Table 2. However, during our optimization of the eye models, we first set the optical power of the eye approximately to 60 D by adjusting the peak plane position defined by Eq. (7) or (8), and then we remove defocus by slightly altering A. V. Goncharov and C. Dainty the VCD table values. The reason for keeping the power of the eye constant is because it is relatively stable between the ages of 20 and 40 years [83], after which there is a shift in the hypermetropic direction [84,85]; that is, the optical power of the eye decreases with age. 5. OPTIMIZATION OF THE GRIN LENS MODELS A. Unbalanced GRIN Lens We start this section with a description of three wide-field schematic eye models having an unbalanced GRIN lens, labeled as 20U, 30U, and 40U. In order to reach a certain level of ocular SA comparable to SA1 or SA2 cases, we optimized all models with reverse ray tracing [86], a technique for duplicating aberrations of optical systems. A recent work on personalized eye models by Navarro et al. [87] showed the reconstruction of the optical system of the eye (including the GRIN lens) from on-axis wavefront measurements using an optimization strategy, which has a strong parallelism with reverse ray tracing. They used a phase plate placed at the pupil plane instead of a reversed eye model. Figure 2 shows the optical system of the Indiana eye (left-hand side) and the optical system under optimization (right-hand side). The SA of the latter is matched to that of the Indiana eye for a 7 mm beam. Off-axis image quality is compared with that of Navarro’s eye model after optimization. We use a conventional plot of RMS wavefront aberration versus the field angle ranging from 0 to 40 deg (see Fig. 3). Ray tracing and optimization were carried out with Zemax-EE optical design software (Focus Software, Inc.). As a result of our extensive search for optimal solutions, we found three models. Their image quality on axis is presented in Fig. 1, which depicts the longitudinal SA as a function of pupil semidiameter h. Table 3 contains optical parameters of the GRIN lens n0 and ⌬n and parameters of its aspheric shape ka and kp. The final values of the optimization parameters are marked by an asterisk. By adjusting n0, we could bring the focal length (or VCD) in line with the table values (Table 2). The SA and the optical power of the lens were set by optimizing parameters ka and kp; the latter defines the peak plane lo- Fig. 3. (Color online) Off-axis wavefront aberrations versus field angle for optimized models of the eye with an unbalanced GRIN lens. Navarro’s eye model is shown for comparison 共 = 0.589 m兲. Vol. 24, No. 8 / August 2007 / J. Opt. Soc. Am. A 2165 cation zm in Eq. (8) and hence the optical power [see Eq. (11)], provided that the value of ⌬n is chosen in advance. We selected ⌬n = 0.035 for all three models. At the final stage, the image defocus was removed by fine-tuning the VCD and the field aberrations were minimized by adjusting the retinal curvature rim. In principle, one could achieve an even better match to the off-axis performance of Navarro’s model by reducing ⌬n below the 0.035 level; nevertheless, smaller values of ⌬n would not be consistent with the latest findings with magnetic resonance imaging [27]. Using four parameters n0, ⌬n, ka, and kp from Table 3 and three parameters d, ra, and rp from Table 2, one can estimate the GRIN lens coefficients from Eqs. (6) and (8), which are listed in Table 4. We present the resulting refractive index variation as a function of distance from the optical axis r, and the axial distance from the anterior surface z in Figs. 4(a) and 4(b), respectively. Figure 5 shows sagittal maps of refractive index variation within the GRIN lens for each model. The diameter of the lens shown is approximately 8 mm; one can easily see a gradual increase in the lens thickness from 3.49 mm (model 20U) to 3.89 mm (model 40U). One of the interesting features of these models is related to the peak plane position, which remains at the same distance of about 5.28 mm from the anterior surface of the cornea. This fact supports a hypothesis of unchanged position of the lens nucleus with age [88]. Comparing refractive index profiles for our models, we can see that the maximum index value nmax in the core and at the anterior surface decreases at a constant rate of −0.004 per decade (a bit slower at the posterior surface) and that the index profiles in the sagittal and equatorial planes gradually flatten out (see Figs. 4 and 5). It is quite likely that such a synchronized refractive index decrease is not a real effect but a consequence of our assumption that iso-indicial contours are coincident with the optical surfaces of the lens. In order to analyze the aberrational characteristics of the lens, we estimate the lens contribution to the primary SA by subtracting the corneal aberrations from the ocular aberrations listed in Table 1; for models 20U, 30U, and 40U we have Z40 = −0.163, −0.132, and −0.109 m, respectively. Our results support earlier findings in several studies [68,71] indicating that the lens partly compensates for SA introduced by the anterior corneal surface and that this compensatory mechanism becomes less efficient with age. Our values of internal SA of the eye (6 mm pupil) are consistent with the empirical data by Alió et al. [59] for the intraocular SA expressed as a function of age (regression line is in the form Z40 = 0.00287A − 0.198). B. Balanced GRIN Lens An unbalanced GRIN lens demonstrates one possible refractive index distribution that provides a realistic amount of ocular SA. Alternatively, we shall now investigate the case for a balanced GRIN lens, which has the same refractive index for its anterior and posterior surfaces. A thorough exploration of multivariable space for the optimal shape of the GRIN lens (with its iso-indicial contours following the optical surfaces) revealed that 2166 J. Opt. Soc. Am. A / Vol. 24, No. 8 / August 2007 A. V. Goncharov and C. Dainty Table 3. Optical Parameters of the Wide-Field Eye Models with Unbalanced (U), Balanced (B), and Simplified (S) GRIN Lensesa Age n0 ⌬n ka kp zm VCD rpp (mm) rim, (mm) Power (D) 20U 30U 40U 20B 30B 40B 20S 30S 40S 1.369* 1.373* 1.377* 1.376 1.376 1.376 1.362 1.362 1.362 0.035 0.035 0.035 0.040 0.040 0.040 0.040 0.040 0.040 −2.8* −2.9* −3.0* 0.0* −1.0* −2.0* −1.0 −1.0 −1.0 2.9* 2.8* 2.7* 2.0* 1.0* 0.0* 0.5 0.5 0.5 1.47 1.67 1.87 1.77 2.00 2.22 1.54* 1.68* 1.81* 16.76* 16.70* 16.62* 16.74* 16.69* 16.62* 16.73* 16.64* 16.53* −7.87 −7.67 −7.47 −14.31* −15.34* −15.93* −7.87 −7.67 −7.47 12.2* 12.0* 12.0* 12.0* 12.0* 12.0* 12.0* 12.0* 11.8* 60.06 60.06 60.14 60.00 59.98 60.02 59.91 60.01 60.19 a Asterisks denote optimized values. Table 4. Refractive Index Coefficients for the Wide-Field Eye Models with Unbalanced (U) and Balanced (B) GRIN Lenses Model n1 n2 n3 n4 n5 n6 n4,2 n5,2 n6,2 20U 30U 40U 20B 30B 40B −0.0017476 0.0000218 0.0429220 −0.0100135 −0.0019289 −0.0000707 −0.0041134 0.0 −0.0003318 −0.0015986 0.0000183 0.0367995 −0.0063555 −0.0015967 −0.0000659 −0.003927 0.0 −0.0002636 −0.0014833 0.0000157 0.0318609 −0.0042843 −0.0013502 −0.0000629 −0.0037343 0.0 −0.0002151 −0.0019829 0.0000246 0.0486997 −0.0168058 0.0012071 −0.0000246 −0.0082314 −0.0026985 −0.0002212 −0.0017358 0.0000188 0.0399587 −0.0099794 0.0 0.0 −0.0107824 −0.0018025 −0.0000753 −0.0015200 0.0000144 0.0326487 −0.0051421 −0.0006203 −0.0000144 −0.0128755 −0.0008624 −0.0000144 there are probably no satisfactory solutions for an index variation range ⌬n ⬎ 0.02, owing to the unrealistically small amount of ocular SA, which was even less than that of the SA1 case. On the other hand, according to recent measurements of the refractive index distribution [27], the typical range for index variation is ⌬n = 0.04, . . . , 0.05. This range is affected by neither age nor intersubject variability of the refractive index n0 at the surface. Consequently, we should adhere to this refractive index range and bring the amount of SA in line with expected values [59]. Interestingly, a study by Peirscionek [29] indicated that earlier schematic eye models [4,9,36] with concentric isoindicial contours following the shape of the lens could not be supported, especially for young lenses. According to Brown [89], density contours obtained from biomicroscopic images do not exactly follow the external shape of the lens. In light of that, we relax our condition for concentric iso-indicial contours for one of the lens surfaces. We chose the posterior surface of the lens, as it has a larger impact on image formation, especially at off-axis angles, due to its distant location from the pupil. Restricting this surface to fulfill the condition of the concentric iso-indicial contours might lead to no feasible solution. The posterior surface is also more convex than the anterior surface (ra / rp is about 1.5), which makes it more sensitive to any changes in shape in terms of balancing the SA of the eye, since the primary SA of a refractive surface is inversely proportional to the cube of its radius of curvature. We now present three wide-field schematic eye models with a balanced GRIN lens, labeled as 20B, 30B, and 40B, having iso-indicial contours less curved than those of the posterior lens surface, which helped to adjust the amount of ocular SA. For our chosen index range ⌬n = 0.04, the radius of curvature for the marginal iso-indicial contour at the posterior vertex (pole) of the lens, denoted as rpp, is about twice the radius of the lens surface. As a result, the refractive index at the posterior surface increases gradually from 1.376 at the pole to 1.39 at 4 mm away from the optical axis. Table 3 lists the optical and geometrical parameters of the GRIN lens n0, ⌬n, ka, kp, and zm and the radius of curvature rpp at the posterior pole used in Eq. (7) as a substitute for rp. The parameter rpp was adjusted so that the VCD is consistent with values in Table 2. In contrast to the optimization of the unbalanced GRIN lens, we selected the same value n0 = 1.376 for all models. By varying the conic constants ka and kp, we could obtain the right amount of SA and the desired optical power of 60 D. The refractive index coefficients are calculated from Eqs. (6) and (7) and are given in Table 4. The LSA of our models with a balanced GRIN lens is presented in Fig. 1. It is clearly seen that plotted curves of the LSA for the 30B and 40B models show a less dramatic change at the edge of the pupil compared with the curves of the 30U and 40U models. This means that the balanced GRIN lens has a smaller amount of high-order SA, which allows us to achieve a better fit to the SA2 case. Table 1 gives the ocular SA. The contribution from the GRIN lens A. V. Goncharov and C. Dainty Vol. 24, No. 8 / August 2007 / J. Opt. Soc. Am. A 2167 Fig. 4. Refractive index profiles in the peak plane (a) and sagittal plane (b) for the 20U, 30U, 40U, 20B, 30B, 40B, 20S, 30S, and 40S models. Fig. 5. Iso-indicial contours following the shape of the crystalline lens. The refractive index values are in increments of 0.002, starting from the anterior surface value n0 and reaching the central contour at 1.411, 1.407, and 1.403 for the 20U, 30U, and 40U models, respectively. 2168 J. Opt. Soc. Am. A / Vol. 24, No. 8 / August 2007 is Z40 = −0.17, −0.14, and −0.12 m for the 20B, 30B, and 40B models, respectively. They display an even larger compensation of the corneal SA than that found in the 20U and 30U models. The RMS wavefront aberrations (units in ) are presented in Fig. 6 together with the aberrations of the Navarro’s eye model for comparison. Similar to the models with an unbalanced lens, wavefront aberrations at 40 deg are slightly higher than that of Navarro’s model. Analyzing the impact of the index range ⌬n on off-axis aberrations, we found that reducing the range ⌬n by half helps to lower the off-axis aberrations but is not sufficient to reach the level of Navarro’s model at 40 deg. Using Eqs. (6) and (7), we present the refractive index variation as a function of distance from the optical axis r and axial distance z in Figs. 4(a) and 4(b), respectively. The sagittal maps of refractive index within the GRIN lens for each model are shown in Fig. 7. The growth of the lens and the internal shift of the core can be easily ob- Fig. 6. (Color online) Off-axis wavefront aberrations versus field angle for optimized 20B, 30B, and 40B models of the eye with a balanced GRIN lens 共 = 0.589 m兲. A. V. Goncharov and C. Dainty served. The diameter of the lens defined by the optical surfaces is about 9.5 mm. C. Simplified GRIN Lens Finally, we investigate the usability of a GRIN lens model with its marginal iso-indicial contours more curved than the optical surfaces of the lens. In order to make the isoindicial contours grow steeper, while at the same time maintaining SA at a realistic level for our chosen index range ⌬n = 0.04, we need to reduce the index coefficient n2 so that it becomes negative. Setting the coefficient n2 to a negative value and using Eqs. (6) leads to a more general case, where the shape of the optical surfaces of the GRIN lens changes form a conicoid to an aspheric of higher order. For that reason, we shall use a simplified single-core GRIN lens with index coefficients defined by Eqs. (9). We present here three models, 20S, 30S, and 40S, optimized by varying the position of the peak plane zm and index coefficient n2 directly. Even though our initial concept of setting the asphericity of the lens surfaces is not applicable for the simplified GRIN lens model, the index coefficient n2 is helpful for adjusting the steepness of the iso-indicial contours, thanks to its direct link to the refractive index profile in the equatorial plane. This makes the coefficient n2 an ideal parameter to regulate the amount of SA in the lens without any changes in the paraxial properties of the lens, since the radii of curvatures ra and rp remain the same. This is also evident from analyzing the contribution Wb2 in Eq. (13). Adjusting the index coefficient n2, we brought the SA of the lens in good agreement with the data by Alió et al. [59]. The optical and geometrical parameters of the GRIN lens n0, ⌬n, ka, kp, and zm are presented in Table 3. The refractive index coefficients are calculated from Eqs. (9) and are listed in Table 5. The asphericity of the marginal anterior and posterior iso-indicial contours calculated from Eq. (10) are approximately 12.7 and 3.4 for the 20S model, 9.0 and 1.3 for the S30 model, and 5.5 and −0.7 for Fig. 7. Iso-indicial contours following the anterior surface of the crystalline lens. The refractive index values are in increments of 0.004, starting from the surface value n0 = 1.376 and reaching the central contour at 1.412 for all models 共nmax = 1.416兲. A. V. Goncharov and C. Dainty Vol. 24, No. 8 / August 2007 / J. Opt. Soc. Am. A the 40S model. For all three models, the marginal isoindicial contours have a steeper shape than the optical surfaces of the lens. As a result, the refractive index at the anterior and posterior surfaces decreases gradually from the pole to the equator. The longitudinal SA of our models with a simplified GRIN lens is presented in Fig. 1. Similar to the models with a balanced lens, the plotted curves of LSA for the 30S and 40S models appear less curved compared with the 30U and 40U models, which indicates a better fit to the SA2 case. The ocular and corneal SAs are presented in Table 1, from which we can estimate the contribution of the GRIN lens: Z40 = −0.166, −0.128, and −0.1048 m for the 20S, 30S, and 40S models, respectively. The RMS wavefront aberrations of these simplified models shown in Fig. 8 more closely resemble aberrations of Navarro’s eye model, especially at 40 deg off axis, while the other models show somewhat higher aberrations at oblique angles. D. Lens Paradox The lens paradox is the phenomenon in which the external surfaces of the crystalline lens become steeper with age [80] without producing any noticeable increase in optical power of the human eye. On the contrary, according to Sounders [84,85], the ocular power decreases; that is, the eye becomes hypermetropic with age, showing a mean power reduction of 2 D between the ages of 30 and 60 years. Grosvenor [90] reanalyzed biometric data from the late 1950s and found a reduction of 0.6 mm in the mean axial length of the eye for a 50+ age group comTable 5. Refractive Index Coefficients for the Wide-Field Eye Models with a Simplified (S) GRIN Lens Model 20S 30S 40S n1 n2 n3 n4 n5 n6 −0.0023783 −0.0000110 0.0584122 −0.0258500 −0.0035000 −0.0002547 −0.0021490 −0.0000106 0.0494670 −0.0159580 0.0001715 0.0001410 −0.0019508 −0.0000090 0.0419020 −0.0085090 −0.0020070 0.0003663 Fig. 8. (Color online) Off-axis wavefront aberrations versus field angle for optimized 20S, 30S, and 40S models of the eye with a simplified GRIN lens 共 = 0.589 m兲. 2169 pared with the third-decade age group. The effect of the gradual decrease in axial length with age might resolve the lens paradox. However, more recent data do not show this effect [77,91]. To explain the lens paradox Koretz and Handelman [92] suggested that with age the effect of increasing curvatures of the lens is precisely balanced by the lens growth along the optical axis (gradual thickening). On the other hand, calculations by Dubbelman and Van der Heijde [78] showed that the thickening of the lens could only partly compensate for its more convex shape and that some additional mechanism for stabilizing the optical power is needed. They also pointed out that Scheimpflug slit images of the crystalline lens used to estimate the lens shape could be distorted due to the refractive properties of the cornea and more significantly due to the GRIN structure of the lens itself; as a result, the effect of lens steepening reported by Brown [80] might not be so dramatic. Since the corneal power does not become weaker with age [60], one of the possible mechanisms resolving the lens paradox is an age-related change in the refractive index distribution in the lens. The optical power of the lens has two distinct components: namely, the refracting power associated with the anterior and posterior surfaces and the power due to the refractive index distribution within the lens. Pierscionek [93] suggested that a slight change in the slope of refractive index in the cortex might compensate the increase in lens curvature and prevent the eye from becoming myopic with age. This hypothesis was shown to be feasible [94]. Using the Wood lens as an approximation, Smith and Pierscionek [95] examined a GRIN lens model with the inner refractive index distribution based on elliptical iso-indicial contours. Considering the biochemistry of the lens, they assumed that the indices at the edge and center of the lens do not change with age. The decrease of lens power was attributed to the gradual steepening of the refractive index profile in the cortex of the lens. An earlier study of biometric data for two different age groups by Hemenger et al. [96] led to a similar conclusion, that subtle changes in the distribution of refractive index within the lens might compensate to a large extent changes in surface curvatures. Another hypothesis proposed to resolve the lens paradox includes a gradual reduction in the index difference between the edge and the center of the lens. There are two possibilities for this case: Either the edge refractive index could increase [97] or the central refractive index could decrease with age [98]. Theoretically, either of these scenarios is possible [99]; however, in reality it is more likely to find several different factors contributing to the lens paradox. The ultimate confirmation of the theoretical modeling of the age-related changes in refractive index distribution has been limited by practical difficulties in measuring the index distribution in the sagittal plane of the lens. The equivalent optical power of the crystalline lens can be found approximately from Eq. (11) or by exact ray tracing. For these two methods, Table 6 gives the optical power of the lens surrounded by the media with refractive index of n0 identical to the surface value of the lens. We can see that Eq. (11) predicts quite accurately the optical power of the lens. Table 6 also shows results for more 2170 J. Opt. Soc. Am. A / Vol. 24, No. 8 / August 2007 A. V. Goncharov and C. Dainty Table 6. Equivalent Optical Power of the GRIN Lens in Media with Refractive Index n = n0 and n = 1.336 (Natural Conditions) Model 20U 30U 40U 20B 30B 40B 20S 30S 40S Fapprox (D) Fexact (D) Fnatural (D) 12.1 12.5 22.1 11.7 12.0 21.5 11.4 11.6 21.0 13.7 13.8 21.9 12.7 12.8 21.3 11.7 11.8 20.6 16.4 16.5 21.8 15.6 15.8 21.3 14.9 15.1 20.8 Fig. 9. Iso-indicial contours within the crystalline lens. The refractive index values are in increments of 0.004, starting from the surface value n0 = 1.362 and reaching the central contour at 1.398 for all models 共nmax = 1.402兲. natural conditions in vivo with surrounding refractive index of 1.336 (vitreous), which mimics the experimental study of isolated crystalline lenses by Jones et al. [27]. According to their average data, the optical power of isolated (unstretched) lenses decreases with age from 25.7 D (20-year age) down to 21.0 D (40-year age). Lenses in our models show somewhat slower decrease of optical power with age, which is likely to be due to their unaccommodated state, whereas isolated lenses lack stretching support from ciliary muscles and therefore assume more convex shape (accommodated state), especially young lenses. The decrease of the equivalent power of the crystalline lens with equatorial radius and thickness was pointed by Perez et al. [100], who used the same data by Koretz et al. [79] to account for age-related changes in the lens shape. Similarly, in a study of 27 human lenses, Glasser and Campbell [101] reported noticeable decrease of optical power with age when no stretching was applied to the lens. However, for artificially stretched isolated lenses, the optical power showed insignificant increase with age for young eyes, probably due to overstretching, since no attempt was made to measure the magnitude of the stretching force, and it was set to the maximum level that produces no visible damage to the lens fibers. All three groups of models of the GRIN lens predict decrease in optical power with age in spite of the fact that the lens radius of curvature increases with age. The changes in refractive index distribution within the lens are responsible for this age effect. Our unbalanced eye models demonstrate another possible compensatory mechanism; namely, the refractive index difference between the edge and the center of the lens remains unchanged, while the maximum and minimum values of the index slowly decrease with age (see Figs. 4 and 9). For the other models, as one can see from Fig. 4(a), relatively small increase in refractive index at the periphery of the lens in the equatorial plane is sufficient to retain the overall optical power of the eye and to slightly reduce the lens power with age. These results support Pierscionek’s hypothesis [93,94] of possible age-related changes in refractive index profile (flattening in the central part) that could potentially prevent the eye from becoming myopic with age. 6. DISCUSSION AND CONCLUSIONS The optical system of the human eye is highly complex due to the multilayered structure of the crystalline lens with distributed refractive index. This feature plays an important role in image formation. We made an attempt to describe the refractive index distribution in the crystalline lens by using two analytical models, which can be easily adapted for age-related changes in the shape of the lens and its optical power. The general model of the GRIN lens has two segments joining at the peak plane. This model fulfills the condition A. V. Goncharov and C. Dainty that marginal iso-indicial contours of refractive index be coincident with the external surfaces of the lens. The GRIN structure is constructed using five geometrical parameters of the lens shape (axial thickness d, radii of curvature ra and rp, and conic constants ka and kp of the external surfaces) and one optical parameter, the refractive index range ⌬n. All parameters of shape are age dependent; therefore we presented their typical values for three age groups (20, 30, and 40 years) derived from various experimental data except for asphericity of the lens surfaces, which are not so well known. The anterior and posterior surface asphericities were kept as free variables during optimization of the models to achieve a realistic amount of ocular SA. In principle, one could vary geometrical parameters to form a particular accommodation state of the lens, although we considered only emmetropic eyes. We bring the optical power of the eye to 60 D by regulating the position of the peak plane zm. The index range ⌬n affects the amount of ocular SA. Therefore, the first step is to select ⌬n and find the asphericity (conic constants ka and kp) of the lens such that ocular SA is in the range of the SA1 and SA2 cases. The resulting models show a gradual change in asphericity of the lens with age. The anterior surface becomes more hyperboloidal, whereas the oblate ellipsoidal posterior surface tends to reduce its asphericity by approaching a spherical shape. This process of restructuring of the lens shape is more evident in models with a balanced GRIN lens. Excessive restructuring might be an artifact of the condition of concentricity of iso-indicial contours with the external surfaces imposed on the models, since it is quite unlikely that the lens undergoes drastic changes during the third and fourth decades. Alternatively, moderate restructuring of the shape indicates that models are more likely to give a plausible solution. In that respect, considering a sequence of age-dependent models as the restructuring process, we can avoid an unrealistic solution at an earlier stage. As a final test of plausibility of the models, we performed ray tracing at oblique angles and compared wavefront aberrations with that of Navarro’s model. The general GRIN and Navarro models exhibit a similar amount of wavefront aberrations in the midperiphery of the field (20 deg off axis). At the far periphery (40 deg off axis), however, wavefront aberrations of the general GRIN models are higher, reaching 9 RMS as compared with 7 RMS for Navarro’s model. To achieve a better agreement with wavefront aberrations of Navarro’s model at far periphery, we abandoned the condition of concentricity of iso-indicial contours and simplified the model of the GRIN lens by reducing it to a single-segment model. This model is constructed using three geometrical parameters d, ra, and rp and three optical parameters such as ⌬n, position of the peak plane zm, and index coefficient n2. In the beginning, we select ⌬n and optimize both the peak plane location zm and surface index n0 to attain 60 D optical power for the whole eye. The index coefficient n2 is used exclusively to control the amount of SA in the lens at the final stage of optimization. As seen from Eq. (11), the optical power does not depend on the index coefficient n2, which is the characteristic feature of the simplified GRIN lens model. It enables Vol. 24, No. 8 / August 2007 / J. Opt. Soc. Am. A 2171 us to use conic constants ka and kp of the lens and to some extent the parameter zm for simultaneous optimization of the wavefront aberration at the field periphery. In contrast to the simplified model, for a given ⌬n, ra, and rp, SA of the general GRIN lens model is uniquely determined by the conic constants ka and kp, with the shape of the iso-indicial contours automatically controlled. The condition of concentricity of the iso-indicial contours makes the general model particular suitable for studies of aberrations at different accommodation states for the eye. Explicit representation of the lens shape together with its iso-indicial contours might add stability to the process of reconstructing the optical system of the eye from experimental measurements of ocular aberrations. The simplified model of the GRIN eye has an additional independent parameter (coefficient n2) influencing the SA of the eye at the expense of losing the direct link between the asphericities of the lens and the marginal iso-indicial contours. Reducing the general GRIN lens description to a single equation [Eq. (1)] changes the shape of conicoid surfaces of the constant refractive index to a more complex high-order aspheric form. The simplicity of using a single equation and the flexibility in fitting aberration both on axis and off axis are the main advantages of the model. We were able to obtain a closer fit of the wavefront aberration of Navarro’s model at the field periphery for all three age-dependent models with no altering of the conic constants, which were selected as ka = −1 and kp = 0.5 to give a realistic shape of the lens. The coefficient n2 was gradually increased (see Table 5) without significant change, which indicates only moderate restructuring of the GRIN lens. The refractive index profile slowly flattens out in the equatorial plane with age, resulting in a reduced optical power of the lens despite its more convex shape. This observation supports the hypotheses [93,94] proposed for resolving the lens paradox through the possible mechanism of restructuring the refractive index distribution in the GRIN lens. An ultimate test of the two GRIN lens models requires real data for ocular aberrations across the field, yet there is a strong evidence to believe that their inclusion in the wide-field schematic models provides a more realistic optical system of the eye. The analysis of the experimental data for ocular parameters is essential for a good starting design prior to optimization of the models. The reverse ray-tracing procedure can also be used for solving the inverse problem of the eye with a GRIN lens. It is worth pointing out a few other features of the derived wide-field schematic eye models that add to the credibility of the proposed method to construct the GRIN lens. All nine schematic eye models are consistent with available data on ocular and intraocular SAs showing partial compensation of the corneal contribution by the GRIN lens. Following the restructuring process of the whole eye, we can also note that this compensatory mechanism becomes less efficient with age, a fact that has been confirmed experimentally [59]. The GRIN lens models allow us to mimick subtle changes in the gradientindex distribution. The refractive index ⌬n and surface index n0 are in the expected range of the crystalline lens [27]. Our thorough analysis [102] of field aberrations such 2172 J. Opt. Soc. Am. A / Vol. 24, No. 8 / August 2007 as coma, astigmatism, and field curvature in the 30S model confirms that its overall performance is in good agreement with experimental findings. A. V. Goncharov and C. Dainty 22. 23. ACKNOWLEDGMENTS This research was supported by Science Foundation Ireland under grant SFI/01/PI.2/B039C. We are grateful to R. Navarro, who is supported by the Spanish CICyT under grant FIS2005-05020-C03-01. 24. 25. REFERENCES 1. 2. 3. 4. 5. 6. 7. 8. 9. 10. 11. 12. 13. 14. 15. 16. 17. 18. 19. 20. 21. A. Gullstrand, “Appendix II,” in Handbuch der Physiologischen Optik, 3rd ed. 1909, J. P. Southall trans., ed. (Optical Society of America, 1924), Vol. 1, pp. 351–352. Y. Le Grand and S. G. El Hage, Physiological Optics (Springer-Verlag, 1980). W. Lotmar, “Theoretical eye model with aspherics,” J. Opt. Soc. Am. 61, 1522–1529 (1971). O. Pomerantzeff, M. Pankratov, G.-J. Wang, and P. Dufault, “Wide-angle optical model of the eye,” Am. J. Optom. Physiol. Opt. 61, 166–176 (1984). I. H. Al-Ahdali and M. A. El-Messiery, “Examination of the effect of the fibrous structure of a lens on the optical characteristics of the human eye: a computer-simulated model,” Appl. Opt. 25, 5738–5745 (1995). A. Popiolek-Masajada, “Numerical study of the influence of the shell structure of the crystalline lens on the refractive properties of the human eye,” Ophthalmic Physiol. Opt. 19, 41–48 (1999). Y.-J. Liu, Z.-Q. Wang, L.-P. Song, and G.-G. Mu, “An anatomically accurate eye model with a shell-structure lens,” Optik (Stuttgart) 116, 241–246 (2005). J. W. Blaker, “Toward an adaptive model of the human eye,” J. Opt. Soc. Am. 70, 220–223 (1980). G. Smith, B. K. Pierscionek, and D. A. Atchison, “The optical modelling of the human lens,” Ophthalmic Physiol. Opt. 11, 359–369 (1991). H.-L. Liou and N. A. Brennan, “Anatomically accurate, finite model eye for optical modeling,” J. Opt. Soc. Am. A 14, 1684–1695 (1997). G. Smith, “The optical properties of the crystalline lens and their significance,” Clin. Exp. Optom. 86, 3–18 (2003). D. A. Atchison and G. Smith, “Continuous gradient-index and shell models of the human lens,” Vision Res. 35, 2529–2538 (1995). A. C. Kooijman, “Light distribution on the retina of a wide-angle theoretical eye,” J. Opt. Soc. Am. 73, 1544–1550 (1983). R. Navarro, J. Santamaria, and J. Bescos, “Accommodation-dependent model of the human eye with aspherics,” J. Opt. Soc. Am. A 2, 1273–1281 (1985). M. J. Howcroft and J. A. Parker, “Aspheric curvatures for the human lens,” Vision Res. 17, 1217–1223 (1977). I. Escudero-Sanz and R. Navarro, “Off-axis aberrations of a wide-angle schematic eye model,” J. Opt. Soc. Am. A 16, 1881–1891 (1999). L. N. Thibos, M. Ye, X. X. Zhang, and A. Bradley, “The chromatic eye: a new model of ocular chromatic aberration,” Appl. Opt. 31, 3594–3600 (1992). M. Ye, X. X. Zhang, L. N. Thibos, and A. Bradley, “A new single-surface model eye that accurately predicts chromatic and spherical aberrations of the human eye,” Invest. Ophthalmol. Visual Sci. 34, 777 (1993). R. Navarro, E. Moreno, and C. Dorronsoro, “Monochromatic aberrations and point-spread functions of the human eye across the visual field,” J. Opt. Soc. Am. A 15, 2522–2529 (1998). D. A. Atchison and D. H. Scott, “Monochromatic aberrations of human eyes in the horizontal visual field,” J. Opt. Soc. Am. A 19, 2180–2184 (1998). D. A. Atchison, “Anterior corneal and internal 26. 27. 28. 29. 30. 31. 32. 33. 34. 35. 36. 37. 38. 39. 40. 41. 42. 43. contributions to peripheral aberrations of human eyes,” J. Opt. Soc. Am. A 21, 335–359 (2004). R. Navarro and M. A. Losada, “Aberrations and relative efficiency of ray pencils in the living human eye,” Optom. Vision Sci. 74, 540–547 (1997). E. Moreno-Barriuso and R. Navarro, “Laser ray tracing versus Hartmann–Shack sensor for measuring optical aberrations in the human eye,” J. Opt. Soc. Am. A 17, 974–985 (2000). J. Liang, B. Grimm, S. Goez, and J. F. Bille, “Objective measurements of wave aberrations of the human eye with the use of a Hartmann–Shack wave-front sensor,” J. Opt. Soc. Am. A 11, 1949–1957 (1994). J. Liang and D. R. Williams, “Aberrations and retinal image quality of the normal human eye,” J. Opt. Soc. Am. A 14, 2873–2883 (1997). T. Salmon, L. N. Thibos, and A. Bradley, “Comparison of the eye’s wave-front aberration measured psychophysically and with the Shack–Hartmann wave-front sensor,” J. Opt. Soc. Am. A 15, 2457–2465 (1998). C. E. Jones, D. A. Atchison, R. Meder, and J. M. Pope, “Refractive index distribution and optical properties of the isolated human lens measured using magnetic resonance imaging (MRI),” Vision Res. 45, 2352–2366 (2005). M. Dubbelman, G. L. Van der Heijde, H. A. Weeber, and G. F. J. M. Vrensen, “Changes in the internal structure of the human crystalline lens with age and accommodation,” Vision Res. 43, 2363–2375 (2003). B. K. Pierscionek, “Refractive index contours in the human lens,” Exp. Eye Res. 64, 887–893 (1997). D. Siedlecki, H. Kasprzak, and B. K. Pierscionek, “Schematic eye with a gradient-index lens and aspheric surfaces,” Opt. Lett. 29, 1197–1199 (2004). W. S. Jagger and P. J. Stands, “A wide-angle gradient index optical model of the crystalline lens and eye of the rainbow trout,” Vision Res. 36, 2623–2639 (1996). W. S. Jagger and P. J. Stands, “A wide-angle gradient index optical model of the crystalline lens and eye of the octopus,” Vision Res. 39, 2841–2852 (1999). S. Nakao, T. Ono, R. Nagata, and K. Iwata, “Model of refractive indices in the human crystalline lens,” Jpn. J. Clin. Ophthalmol. 23, 903–906 (1969). B. K. Pierscionek, D. Y. C. Chan, J. P. Ennis, G. Smith, and R. C. Augusteyn, “Nondestructive method of constructing three-dimensional gradient index models for crystalline lenses: 1 theory and experiment,” Am. J. Optom. Physiol. Opt. 65, 481–491 (1988). B. K. Pierscionek and D. Y. C. Chan, “Refractive index gradient of human lenses,” Optom. Vision Sci. 66, 822–829 (1989). D. Y. C. Chan, J. P. Ennis, B. K. Pierscionek, and G. Smith, “Determination and modeling of 3-D gradient refractive indices in crystaline lenses,” Appl. Opt. 27, 926–931 (1988). P. P. Fagerholm, B. T. Philipson, and B. Lindstrom, “Normal human lens, the distribution of protein,” Exp. Eye Res. 33, 615–620 (1981). B. K. Pierscionek, “Variations in refractive index and absorbance of 679 nm light with age and cataract formation in human lenses,” Exp. Eye Res. 60, 407–414 (1995). B. K. Pierscionek, “Surface refractive index of the eye lens determined with an optic fiber sensor,” J. Opt. Soc. Am. A 10, 1867–1871 (1993). B. A. Moffat, D. A. Atchison, and J. M. Pope, “Aged-related changes in refractive index distribution and power of the human lens as measured by magnetic resonance microimaging in vitro,” Vision Res. 42, 1683–1693 (2002). G. Smith and D. A. Atchison, “Equivalent power of the crystalline lens of the human eye: comparison of methods of calculation,” J. Opt. Soc. Am. A 14, 2537–2546 (1997). G. Smith and D. A. Atchison, “The gradient index and spherical aberration of the lens of the human eye,” Ophthalmic Physiol. Opt. 21, 317–326 (2001). P. J. Sands, “Third-order aberrations of inhomogeneous lens,” J. Opt. Soc. Am. 60, 1436–1443 (1970). A. V. Goncharov and C. Dainty 44. 45. 46. 47. 48. 49. 50. 51. 52. 53. 54. 55. 56. 57. 58. 59. 60. 61. 62. 63. 64. 65. 66. 67. 68. Y. Huang and D. T. Moore, “Human eye modeling using a single equation of gradient index crystalline lens for relaxed and accommodated states,” presented at the International Optical Design Conference, Vancouver, British Columbia, Canada, June 4–8, 2006, paper MDI. M. Koomen, R. Tousey, and R. Scolnik, “The spherical aberration of the eye,” J. Opt. Soc. Am. 39, 370–376 (1949). A. Ivanoff, “About the spherical aberration of the eye,” J. Opt. Soc. Am. 46, 901–903 (1956). T. Jenkins, “Aberrations of the eye and their effects on vision: part 1,” Br. J. Physiol. Opt. 20, 59–91 (1963). M. Millodot and J. Sivak, “Contribution of the cornea and lens to the spherical aberration of the eye,” Vision Res. 19, 685–687 (1979). H.-L. Liou and N. A. Brennan, “The prediction of spherical aberration with schematic eyes,” Ophthalmic Physiol. Opt. 16, 348–354 (1996). L. N. Thibos, M. Ye, X. Zhang, and A. Bradley, “Spherical aberration of the reduced schematic eye with elliptical refracting surface,” Optom. Vision Sci. 74, 548–556 (1997). R. L. Woods, A. Bradley, and D. A. Atchison, “Monocular diplopia caused by ocular aberrations and hyperopic defocus,” Vision Res. 36, 3597–3606 (1996). J. Porter, A. Guirao, I. G. Cox, and D. R. Williams, “Monochromatic aberrations of the human eye in a large population,” J. Opt. Soc. Am. A 18, 1793–1803 (2001). L. N. Thibos, X. Hong, A. Bradley, and X. Cheng, “Statistical variations of aberration structure and image quality in a normal population of healthy eyes,” J. Opt. Soc. Am. A 19, 2329–2348 (2002). J. C. He, J. Gwiazda, F. Thorn, and R. Held, “Wave-front aberrations in the anterior corneal surface and the whole eye,” J. Opt. Soc. Am. A 20, 1155–1163 (2003). G. Smith, M. J. Cox, R. Calver, and L. F. Garner, “The spherical aberration of the crystalline lens of the human eye,” Vision Res. 41, 235–243 (2001). R. I. Calver, M. J. Cox, and D. B. Elliott, “Effect of aging on the monochromatic aberrations of the human eye,” J. Opt. Soc. Am. A 16, 2069–2078 (1999). P. Artal, E. Berrio, A Guirao, and P. Piers, “Contribution of the cornea and internal surfaces to the change of ocular aberrations,” J. Opt. Soc. Am. A 19, 137–143 (2002). S. Amano, Y. Amano, S. Yamagami, T. Miyai, K. Miyata, T. Samejima, and T. Oshika, “Age-related changes in corneal and ocular higher-order wavefront aberrations,” Am. J. Ophthalmol. 137, 988–992 (2004). J. L. Alió, P. Schimchak, H. P. Negri, and R. Montés-Micó, “Crystalline lens optical dysfunction through aging,” Ophthalmology 112, 2022–2029 (2005). A. K. C. Lam and W. A. Douthwaite, “The aging effect on the central posterior corneal radius,” Ophthalmic Physiol. Opt. 20, 63–69 (2000). C. Edmund, “Posterior corneal curvature and its influence on corneal dioptric power,” Acta Ophthalmol. 72, 715–720 (1994). A. Guirao, M. Redondo, and P. Artal, “Optical aberrations of the human cornea as a function of age,” J. Opt. Soc. Am. A 17, 1697–1702 (2000). A. Guirao and P. Artal, “Corneal wave aberration from videokeratography: accuracy and limitations of the procedure,” J. Opt. Soc. Am. A 17, 955–965 (2000). M. Sheridan and W. A. Douthwaite, “Meridional variations of corneal shape,” Ophthalmic Physiol. Opt. 9, 235–238 (1989). S. Aoshima, T. Nagata, and A. Minakata, “Optical characteristics of oblique incident rays in pseudophakic eyes,” J. Cataract Refractive Surg. 30, 471–477 (2004). M. Guillon, P. M. Lydon, and C. Wilson, “Corneal topography: a clinical model,” Ophthalmic Physiol. Opt. 6, 47–56 (1986). M. Dubbelman, H. A. Weeber, R. G. L. van der Heijde, and H. J. Volker-Dieben, “Radius and asphericity of the posterior corneal surface determined by corrected Scheimpflug photography,” Acta Ophthalmol. Scand. 80, 379–383 (2002). P. Artal and A. Guirao, “Contributions of the cornea and Vol. 24, No. 8 / August 2007 / J. Opt. Soc. Am. A 69. 70. 71. 72. 73. 74. 75. 76. 77. 78. 79. 80. 81. 82. 83. 84. 85. 86. 87. 88. 89. 90. 2173 the lens to the aberrations of the human eye,” Opt. Lett. 23, 1713–1715 (1998). J. C. He, J. Gwiazda, F. Thorn, R. Held, and W. Huang, “Change in corneal shape and corneal wave-front aberrations with accommodation,” J. Vision 3, 456–463 (2003). L. Wang, E. Dai, D. D. Koch, and A. Nathoo, “Optical aberrations of the human anterior cornea,” J. Cataract Refractive Surg. 29, 1514–1521 (2003). J. E. Kelly, T. Mihashi, and H. C. Howland, “Compensation of corneal horizontal/vertical astigmatism, lateral coma, and spherical aberration by internal optics of the eye,” J. Vision 4, 262–271 (2004). R. Navarro, L. González, and J. L. Hernández, “Optics of the average normal cornea from general and canonical representations of its surface topography,” J. Opt. Soc. Am. A 23, 219–232 (2006). A. Lleó, A. Marcos, M. Calatayud, L. Alonso, S. M. Rahhal, and J. A. Sanchis-Gimeno, “The relationship between central corneal thickness and Goldmann applanation tonometry,” Clin. Exp. Optom. 86, 104–108 (2003). A. K. C. Lam and J. Chan, “Corneal thickness at different reference points from Orbscan II system,” Clin. Exp. Optom. 86, 230–234 (2003). C. A. Cook and J. F. Koretz, “Acquisition of the curves of the human crystalline lens from slit lamp images: an application of the Hough transform,” Appl. Opt. 30, 2088–2099 (1991). M. Dubbelman, G. L. Van der Heijde, and H. A. Weeber, “The thickness of the aging human lens obtained from corrected Scheimpflug images,” Optom. Vision Sci. 78, 411–416 (2001). J. F. Koretz, S. Strenk, L. M. Strenk, and J. L. Semmlow, “Scheimpflug and high-resolution magnetic resonance imaging of the anterior segment: a comparative study,” J. Opt. Soc. Am. A 21, 346–354 (2004). M. Dubbelman and G. L. Van der Heijde, “The shape of the aging human lens: curvature, equivalent refractive index and the lens paradox,” Vision Res. 41, 1867–1877 (2001). J. F. Koretz, C. A. Cook, and P. L. Kaufman, “Aging of the human lens: changes in lens shape at zero-diopter accommodation,” J. Opt. Soc. Am. A 18, 265–272 (2001). N. Brown, “The change in lens curvature with age,” Exp. Eye Res. 19, 175–183 (1974). S. Stenström, “Investigation of the variation and the correlation of the optical elements of human eye,” Am. J. Optom. Arch. Am. Acad. Optom. 25, 340–350 (1948). C. S. Yu, D. Kao, and C. T. Chang, “Measurements of the length of the visual axis by ultrasonography in 1789 eyes,” Chin. J. Ophthal. 15, 45–47 (1979). T. Grosvenor, “Changes in spherical refraction during the adult years,” in Refractive Anomalies. Research and Clinical Applications, T. Grosvenor and M. C. Flom, eds. (Butterworth-Heinemann, 1991), pp. 131–145. H. Saunders, “Age-dependence of human refractive errors,” Ophthalmic Physiol. Opt. 1, 159–174 (1981). H. Saunders, “A longitudinal study of the age dependence of human ocular refraction. 1. Age-dependent changes in the equivalent sphere,” Ophthalmic Physiol. Opt. 6, 343–344 (1986). J. L. Rayces and M. Rosete-Aguilar, “Optical design procedure for duplicating wavefront errors of an optical instrument,” Opt. Eng. (Bellingham) 39, 1768–1775 (2000). R. Navarro, L. González, and J. L. Hernández-Matamoros, “On the prediction of optical aberrations by personalized eye models,” Optom. Vision Sci. 83, 371–381 (2006). J. F. Koretz, C. A. Cook, and J. R. Kuszak, “The zones of discontinuity in the human lens: development and distribution with age,” Vision Res. 34, 2955–2962 (1994). N. Brown, “The change in shape and internal form of the lens of the eye on accommodation,” Exp. Eye Res. 15, 441–459 (1973). T. Grosvenor, “Reduction in axial length with age: an emmetropizing mechanism for the adult eye?” Am. J. Optom. Physiol. Opt. 64, 657–663 (1987). 2174 91. 92. 93. 94. 95. 96. 97. J. Opt. Soc. Am. A / Vol. 24, No. 8 / August 2007 C. S. Ooi and T. Grosvenor, “Mechanisms of emmetropization in the aging eye,” Optom. Vision Sci. 72, 60–66 (1995). J. F. Koretz and G. H. Handelman, “The lens paradox and image formation in accommodating human eyes,” Top. Aging Res. Eur. 6, 57–64 (1986). B. K. Pierscionek, “Presbyopia—effect of refractive index,” Clin. Exp. Optom. 73, 23–30 (1990). G. Smith, D. A. Atchison, and B. K. Pierscionek, “Modeling the power of the aging human eye,” J. Opt. Soc. Am. A 9, 2111–2117 (1992). G. Smith and B. K. Pierscionek, “The optical structure of the lens and its contribution to the refractive status of the eye,” Ophthalmic Physiol. Opt. 18, 21–29 (1998). R. P. Hemenger, L. F. Garner, and C. S. Ooi, “Changes with age of the refractive index gradient of the human ocular lens,” Invest. Ophthalmol. Visual Sci. 36, 703–707 (1995). L. F. Garner, C. S. Ooi, and G. Smith, “Refractive index of A. V. Goncharov and C. Dainty the crystalline lens in young and aged eyes,” Clin. Exp. Optom. 81, 145–150 (1998). 98. B. A. Moffat, D. A. Atchison, and J. M. Pope, “Explanation of the lens paradox,” Optom. Vision Sci. 79, 148–150 (2002). 99. J. F. Koretz and C. A. Cook, “Aging of the optics of the human eye: lens refraction models and principal plane locations,” Optom. Vision Sci. 78, 396–404 (2001). 100. M. V. Perez, C. Bao, M. T. Flores-Arias, M. A. Rama, and C. Gomez-Reino, “Description of gradient-index crystalline lens by a first-order optical system,” J. Opt. A, Pure Appl. Opt. 7, 103–110 (2005). 101. A. Glasser and M. C. W. Campbell, “Presbyopia and the optical changes in the human crystalline lens with age,” Vision Res. 38, 209–229 (1998). 102. A. V. Goncharov and C. Dainty are currently preparing a manuscript to be called “Aberrations of chromatic widefield schematic eye model with a GRIN lens.” (alexander.goncharov@nuigalway.ie)