Acetanilide Recrystallization Lab: Purification & Melting Point
advertisement
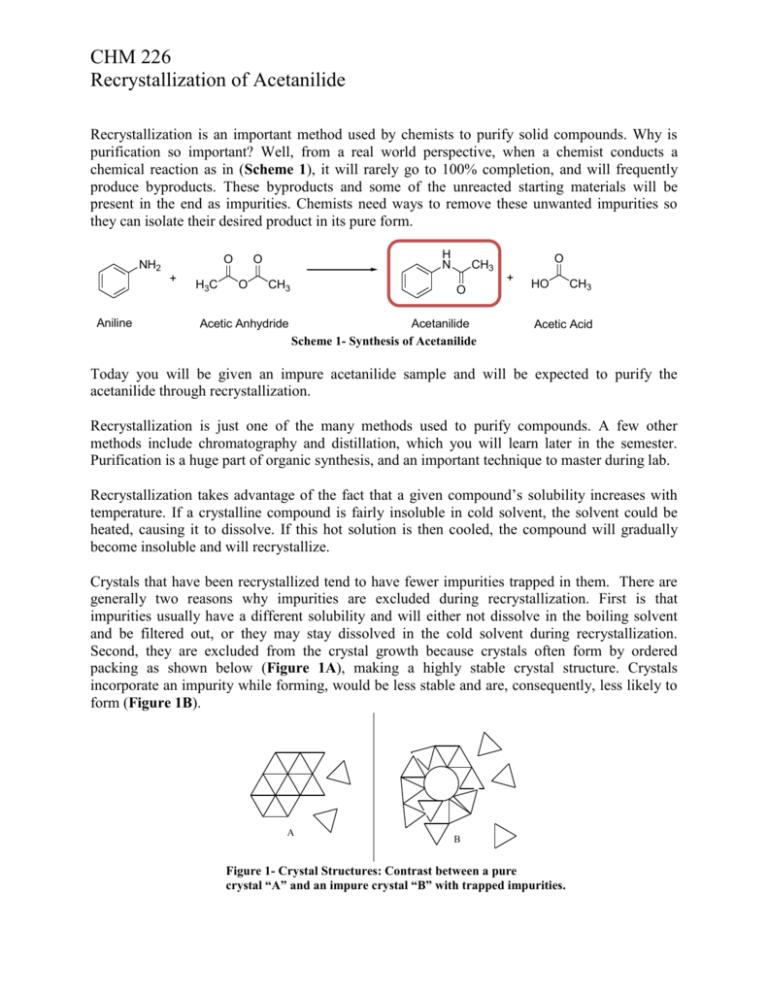
CHM 226 Recrystallization of Acetanilide Recrystallization is an important method used by chemists to purify solid compounds. Why is purification so important? Well, from a real world perspective, when a chemist conducts a chemical reaction as in (Scheme 1), it will rarely go to 100% completion, and will frequently produce byproducts. These byproducts and some of the unreacted starting materials will be present in the end as impurities. Chemists need ways to remove these unwanted impurities so they can isolate their desired product in its pure form. O NH2 + Aniline H3C H N O O + CH3 Acetic Anhydride O CH3 O Acetanilide Scheme 1- Synthesis of Acetanilide HO CH3 Acetic Acid Today you will be given an impure acetanilide sample and will be expected to purify the acetanilide through recrystallization. Recrystallization is just one of the many methods used to purify compounds. A few other methods include chromatography and distillation, which you will learn later in the semester. Purification is a huge part of organic synthesis, and an important technique to master during lab. Recrystallization takes advantage of the fact that a given compound’s solubility increases with temperature. If a crystalline compound is fairly insoluble in cold solvent, the solvent could be heated, causing it to dissolve. If this hot solution is then cooled, the compound will gradually become insoluble and will recrystallize. Crystals that have been recrystallized tend to have fewer impurities trapped in them. There are generally two reasons why impurities are excluded during recrystallization. First is that impurities usually have a different solubility and will either not dissolve in the boiling solvent and be filtered out, or they may stay dissolved in the cold solvent during recrystallization. Second, they are excluded from the crystal growth because crystals often form by ordered packing as shown below (Figure 1A), making a highly stable crystal structure. Crystals incorporate an impurity while forming, would be less stable and are, consequently, less likely to form (Figure 1B). A B Figure 1- Crystal Structures: Contrast between a pure crystal “A” and an impure crystal “B” with trapped impurities. CHM 226 Recrystallization of Acetanilide In order to make recrystallization occur, a few guidelines must be followed when choosing a solvent to recrystallize with. 1. Generally like dissolves like. Polar solvents tend to dissolve polar compounds, etc. 2. A solvent (or mixture of solvents) should not dissolve the compound you are purifying at room temperature. If the compound dissolves at room temperature, your compound will not recrystallize back out of the solvent at room temperature. 3. A solvent should dissolve the compound you are purifying at the solvent’s boiling point. If the compound does not go into solution when boiling, you will not be able to recrystallize. 4. The boiling point of the solvent must be lower than the melting point of the compound you are purifying. If boiling point of the solvent is greater than the melting point of the compound, the compound will melt into an oil (oil out) and will likely not be miscible with the solvent. 5. A solvent should at lower temperatures release a lot of crystals from solution. Once a successful recrystallization has been carried out, there should be lots of crystals sitting in a cold solvent. The last step is to isolate the purified crystals by using vacuum filtration. In this experiment, you will have to choose the best recrystallizing solvent based on the above guidelines. After recrystallizing your compound, you will vacuum filter the purified crystals, and then calculate % recovery. . A melting point of the recrystallized compound will be taken in order to determine the purity. A melting point is taken by loading a small amount of solid crystals into a tiny hollow glass tube, called a capillary. The capillary is then placed inside a melting point apparatus, which contains a heat source and a magnifying glass on top for viewing. As the apparatus starts to heat, you can view the crystals to see when they start to liquefy. A pure compound will exhibit a very sharp melting point with a range spanning only a few degrees Celsius; however, if there are impurities present, the impurities will cause the compound to start melting prematurely at a lower temperature, and will often cause the range of melting to be broad (greater than 5 degrees Celsius). Purifying Acetanilide by Recrystallization Reference: Wigal, C. T. Signature Labs Series: Chemistry Lab Experiments CHEM 226; Jefferes, J., Ed.; Cengage Learning: Mason, OH, 2008; p 29-36 Data Table: Have a copy of this in your notebook prior to the beginning of lab. Your data table should contain columns for the weight of your crude acetanilide, the weight of your filter paper, the weight of your purified acetanilide + filter paper, the weight of pure acetanilide, and % recovery, and the literature melting point of acetanilide. CHM 226 Recrystallization of Acetanilide Equipment: 50mL beaker 100mL beaker w/ boiling solvent Sand Bath Hot Plate Figure 2- Recrystallization Apparatus Eyepiece Power Switch Temperature Readout Stop Button Set Button Start Button Figure 3- Melting Point Apparatus CHM 226 Recrystallization of Acetanilide Procedure: To start, you will have four different recrystallizing solvents to choose from water, acetone, ethanol, and petroleum ether. You will want to first test all four of these solvents to ascertain which solvent is most suitable for your recrystallization. To do this, take four test tubes and label them acetone, water, ethanol, and pet ether. Pipette about 2 mL of each solvent in the corresponding test tubes and add a small scoop of acetanilide to each with your spatula. Stir the solutions by shaking the tubes and record in your notebook for each solvent whether the acetanilide is soluble or insoluble. Next take each test tube that the acetanilide did not dissolve in and heat the test tubes by placing them in a sand bath, and turning the sand bath on high heat. When the solvents start to boil, stir the solutions and record in your notebook whether the acetanilide was soluble or insoluble. Let the test tubes cool to room temperature. Finally, make an ice bath, by putting ice in a 250 mL beaker and filling the beaker up with water. Place the tests tubes in the ice bath. Let them sit for 5 minutes and write in your notebook whether recrystallization occurs. Based on these quick tests select the solvent you will use to recrystallize acetanilide and record it in your notebook. Check with your TA to make sure you select the right solvent. Next, you can start your recrystallization! Weigh out 500 mg of crude acetanilide into a 50 mL beaker. Record the exact weight in your notebook. You should warm your sand bath again by turning the hot plate on medium-high. Measure out 20-30 mL of your solvent into a 100 mL beaker and place it on your hot plate. Once the solvent starts boiling, pipette some of the hot solvent into the 50 mL beaker with your crude acetanilide in it. The solvent cools down slightly while you are pipetting, so let it warm up to boiling again by swirling the beaker gently in the warm sand. (Figure 2) DO NOT leave the beaker sitting in the sand, or the acetanilide will oil out. Keep adding a pipette of hot solvent and warming in the sand to boiling until all of the crystals have dissolved. Next, remove the beaker of dissolved crystals from the heat and let it cool. Once it reaches room temperature, cool it further by placing it in your ice bath for 5-10 minutes. White crystals should start to fall out of solution as it cools, if no crystals fall out try scratching the bottom of the flask with a glass stirring rod or metal spatula. While it is cooling, measure out 2 mL of your recrystallizing solvent into a test tube and cool it in your ice bath. This will be used to rinse your product when filtering. Set up your vacuum filtration apparatus using a 125 mL filter flask, a Hirsch funnel, and a filter paper. Weigh the filter paper and record it in your lab notebook. Turn the vacuum pump on and filter the purified acetanlide. Use your metal spatula to scrape the crystals out of the 50 mL beaker into the Hirsch funnel, and the cold solvent to rinse any CHM 226 Recrystallization of Acetanilide remaining crystals out of the beaker. Let the vacuum pump run for 10-15 minutes. Once the crystals are dry on the filter paper, carefully weigh the filter paper and pure acetanilide. Finally before leaving lab, you will need to obtain a melting point. Taking a Melting Point (See Figure 3) Start by spreading your crystals on a watch glass. Take a melting point capillary, and tap the open end down into your crystals to load a few crystals inside the capillary. Turn the capillary upside down and lightly tap it on the bench top to load the crystals into the bottom of the capillary. Keep repeating this until 1-2 mm of crystals are in the bottom of your capillary. Plug the melting point apparatus in. Turn the power on by pressing the switch on the left hand side of the instrument. If you do not know the literature melting point of your compound, start here. Insert your sample and start by setting your plateau very high ~150C, you set the plateau by holding down on the “Set” button and adjusting the temperature. Once you have set the plateau temperature, insert your sample and press start. The instrument will rapidly heat up to the plateau temperature. YOU MUST WATCH YOUR SAMPLE CLOSELY THE WHOLE TIME IT IS HEATING, or you might miss the approximate melting point. When your sample starts to liquefy look at what temperature the instrument is at, and record this as your “orientation melting point”, just so you know the approximate range of melting during rapid heating. Press the “Stop” button to let the instrument cool down again. To fine tune this melting point you will use a second melting point capillary with your sample to slowly check the melting point. Continue in the next step. If your know the literature melting point of your sample or the “orientation melting point,” start here. Set the plateau temperature at 15 C below the literature or “orientation” melting point by holding the “Set” button. Insert your sample in the instrument and press “Start”. Let the instrument heat up until the plateau light comes one. Then press “Start” again and the ramping light will come on. This will slowly ramp the temperature. Keep an eye on your sample and record the exact range your compound melts. Once your compound has finished melting press the “Stop” button, and then turn the instrument off by flipping the power button. CHM 226 Recrystallization of Acetanilide Pre-lab Questions 1.) Let’s say you wanted to recrystallize compound “A”. Compound “A” is a semi-polar compound with a melting point of 87 oC. There are four possible recrystallizing solvents to choose from: acetone, water, dimethylsulfoxide, and hexane. Based on all of the information given and the following flow chart, what ONE solvent would be best suited for the recrystallization of “A”. Give a short explanation of why that solvent will work. Impure Compound “A” Room Temperature Solvent Soluble in: DMSO Insoluble in: DMSO, Hexane, Water Boiling Solvent Soluble in: Acetone & Water Insoluble in: Hexane 2.) Compounds, even though they do not look visibly soluble, can still be slightly soluble in solvents at room temperature. If acetanilide’s solubility is 4mg/L in your solvent at room temperature how much acetanilide would you expect to lose if you use 9 mL of solvent to dissolve 0.450 g of acetanilide, and rinse with 3 mL of solvent during filtration? 3.) Why is it bad to use too much solvent when dissolving the compound initially? Why is it important to use ice-cold solvent when rinsing recrystallized product during filtration? Post-lab Questions 1.) Why does an impure compound start melting at a lower temperature? 2.) Show your calculation for % recovery. Is this percentage what you were expecting? Why?




