Hernandez 1 UNKNOWN BACTERIA #15 REPORT
advertisement

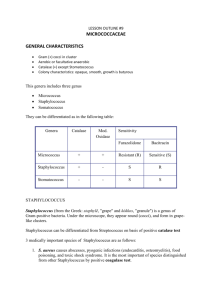
Hernandez 1 UNKNOWN BACTERIA #15 REPORT- STAPHYLOCOCCUS AUREUS BIOLOGY 211- GENERAL MICROBIOLOGY AND PUBLIC HEALTH LABORATORY SAN FRANCISCO STATE UNIVERSITY PROFESSOR LANCE LUND MAY 10, 2012 WRITTEN BY: ALMA HERNANDEZ Hernandez 2 UNKNOWN #15- STAPHYLOCOCCUS AUREUS PURPOSE To identify unknown bacterium #15 using a series of tests and observation techniques. PROCEDURES/RESULTS In order to identify unknown bacterium #15, several tests and observation techniques were necessary. First, the colony characteristics such as the color, size, shape, margin, elevation and texture were to be observed and recorded. BHI media was inoculated with liquid broth of unknown #15 and incubated at 37 degrees C for 48 hours. After the 48 hour incubation period, BHI media appeared to have numerous circular colonies with curved edges and a golden brown color. Also, on the media were four small patches of mold, some with small branches. BHI broth was also inoculated with liquid broth of unknown #15 to determine whether there would be microbial growth. The broth appeared to be turbid with a small pellicate at the bottom of the broth indicating microbial growth (See Figure 1). Next, cellular characteristics such as gram stain reaction, cell size, morphology and arrangement were observed and identified. BHI media was inoculated with liquid broth of unknown #15 and incubated at 37 degrees Celsius for 48 hours. After the incubation period, a small sample of a bacterial colony was placed on a slide, heat fixed and gram stained. The gram stained slide was then observed under the microscope at 1000x magnification. Purple coccus shaped bacteria were observed. The bacteria appeared to be arranged in grape-like clusters and measured out at about one micrometer in length. Based on the observations, it was determined that unknown bacterium Hernandez 3 #15 is a gram positive coccus. In addition, a catalase test was also done in order to determine the presence of the enzyme catalase. A small sample of a live bacterial colony of unknown #15 from the BHI media was placed on a slide. Two small drops of 3% hydrogen peroxide H2O2 were added. Gas bubbles were observed, resulting in a catalase positive test result. Unknown #15 contains the enzyme catalase and is able to convert H2O2 into water and Oxygen. Similarly, an oxidase test was also done in order to test for the presence of the enzyme oxidase. A live colony of unknown #15 was placed on filter paper. Two drops of oxidase reagent were added to the colony. 15-30 seconds later, there was no change in the appearance of the colony, producing a negative test result. Had it been a positive result, the colony would have turned purple within seconds. Several environmental factors play a big role in cell growth or death. In order to narrow the results down further, tests regarding temperature, pH, osmotic pressure and oxygen requirement were also done. By cultivating anaerobes, students were able to determine the oxygen requirement, which serves as a good test to identify the unknown bacterium. Two BHI media plates were inoculated with liquid broth of unknown #15. They were each incubated at 37 degrees C for 48 hours. One plate was placed inside a Brewer’s Jar, which only grows anaerobes, and the other one was not. Separately, thyoglycollate broth was also inoculated with liquid broth of unknown #15 and incubated at 37 degrees C for 48 hours. After the 48 hour incubation period, plates and broth were observed and results were tabulated. There was slight growth of unknown #15 in the BHI plate that was placed in the Brewer’s jar. There was also thick growth in the BHI plate that was incubated with Oxygen. The thyoglycollate broth showed significant growth all throughout as well (See Figure 2). These results indicate that unknown #15 is indeed a Hernandez 4 facultative anaerobe because it was able to grow in an oxygen rich environment as well as an oxygen deprived environment. Unknown #15 can use both aerobic and anaerobic fermentation pathways for generation of ATP. Additionally, tests were done to determine the effects of pH and temperature on unknown #15. For temperature, four different BHI aliquot tubes were inoculated with 50mL of unknown #15 and incubated at four different temperatures for 48 hours. One was incubated at 10 degrees C, one at 25 degrees C, one at 37 degrees C and one at 55 degrees C. Similarly, four separate BHI aliquot tubes were inoculated with 50mL of unknown #15. Each tube contained a different pH level. One had a pH of 3, one of 5, one of 7 and one of 9. All four of these tubes were incubated at 37 degrees C for 48 hours. After the 48 hour incubation period, all tubes were observed and the broth of each tube was placed in a spectrophotometer to measure the turbidity and determine the degree of microbial growth. Unknown bacterium #15 had optimum growth at about 37 degrees C and at a pH level of about 9 (See Figures 2 and 3). Osmotic pressure is also good at determining the type of bacterium present because not all bacteria can withstand high concentrations of salt. In order to identify the effects of osmotic pressure, four agar plates with different salt concentrations were inoculated with broth of unknown #15 and incubated at 37 degrees C. One plate had 1% NaCl, one had 5% NaCl, one had 10% NaCl and one had 25% Hernandez 5 NaCl. After the 48 hour incubation period, results were observed and recorded. Although all four plates indicated some growth of unknown #15, the growth was most abundant on agar plate with 1% NaCl concentration. Clearly, unknown bacterium #15 can withstand salty environments. Since unknown #15 indicated to be a gram-positive coccus shaped bacterium, further testing was necessary in order to differentiate the species. A mannitol salt agar plate was used because it is considered both a selective and differential media. It contains 7.5% NaCl, mannitol and a phenol red indicator. The plate was inoculated with broth of unknown #15 and incubated at 37 degrees C for 48 hours. After the incubation, the plate appeared to have significant growth and the color changed from red to completely yellow, indicating mannitol fermentation. Species such as Staphylococcus Aureus and Staphylococcus Saprophyticus produce acids during mannitol fermentation, which in turn can change the color of the media from red to yellow (Lab Manual 264). In order to determine whether unknown #15 was a species of S.aureus or S.saprophyticus, a blood agar plate and an mStaphylococcus broth were used. The blood agar plate with 5% sheep’s blood was inoculated with a live colony of unknown #15 from the mannitol salt agar plate. On one side of the plate, a small disc of the antibiotic Novobiocin was also placed in order to test for sensitivity versus resistance. The plate was then incubated at 37 degrees C for 48 hours in order to detect hemolysis or the lysis of red blood cells by bacterial toxins. The m-Staphyloccocus broth was Hernandez 6 also inoculated with a live colony and incubated to test for the presence of staphylococci and coagulation of plasma. After the 48 hour incubation period, the blood agar plate and the broth were observed and results were recorded. On the blood agar plate, there was clear beta hemolysis of the red blood cells and clear sensitivity to Novobiocin. The m-Staphylococcus Broth also indicated coagulation of plasma (See Figure 4). DISCUSSION With the use of BHI media, unknown #15 was able to be cultivated and its physical characteristics were observed and determined. Upon observation of the colony and cellular characteristics, unknown #15 was identified as a gram positive coccus. Therefore, all gram negative rods and spirochetes were eliminated. Additionally, testing for the presence of catalase and oxidase allowed for further identification. Since unknown #15 tested positive for the catalase test, all bacteria not containing the enzyme catalase were eliminated. Also, unknown #15 tested negative for the oxidase test, thus all bacteria containing the enzyme oxidase were eliminated as well. Further testing regarding environmental factors helped narrow down to more specific bacterial candidates. Incubating plates in oxygen rich and oxygen deprived environments indicated that unknown #15 was a facultative anaerobe because growth was seen on both plates. Therefore, all obligate anaerobes and obligate aerobes were eliminated. Moreover, results for temperature effects on microbial growth indicated that the optimal growth for unknown #15 was at about 37 degrees C. This ruled out thermophiles and psychrophiles as possible candidates. In addition, results for pH effects on growth determined that unknown #15 had optimum growth at pH level 9, thus eliminating acidophiles. Also, in the osmotic pressure test, unknown #15 demonstrated growth in all four salt concentrations. Since unknown #15 proved to be an Hernandez 7 osmotolerant bacterium, all bacteria unable to withstand high salt concentrations such as nonosmotolerant organisms, were eliminated. With the help of a mannitol salt agar, blood agar and an m-Staphylococcus broth, further investigation helped in the process of identification. The results of mannitol salt agar plate indicated that unknown #15 was a Beta Hemotytic bacteria, therefore eliminating all Alpha Hemolytic bacteria. Also, mannitol fermentation on the salt agar eliminated all bacteria unable to ferment mannitol. Unknown #15 was also able to coagulate plasma and was sensitive to Novobiocin, thus indicating that it is Staphylococcus Aureus. According to Bergey’s Manual of Determinative Bacteriology, Staphylococcus Aureus is a “gram positive, non-motile, nonsporing, facultative anaerobe” that measures at about 0.5-1.5 micrometers in diameter (544). It is usually arranged in pairs or irregular grape-like clusters. Typically, it is catalase positive and catalase negative (544). Its optimum temperature is about 30-37 degrees C and can grow in an environment of about 10% salt concentration (544). Also, it is “mainly associated with the skin and mucous membranes of warm-blooded vertebrates but are often isolated from food products, dust and water” (544). While most strains of S.aureus are harmless, some can be “opportunistic pathogens” and can cause disease in humans and animals (544). S.aureus can be responsible for a number of infections such as “minor skin lesions” and even major wound infections like pneumonia, endocarditis and osteomyelitis (Lab Manual 263). Furthermore, S.aureus has been a “nosocomial opportunist” causing widespread infections in hospitals and now it is being brought out into the community causing infections such as Methicillin-Resistant S.aureus or MRSA for short (Lab Manual 263). In an article titled “Methicillin-Resistant S. aureus Infections among Patients in the Emergency Department”, Gregory Moran explains that MRSA is rapidly emerging as a Hernandez 8 “community pathogen” (1). He also says that it is threatening to “change the practice of outpatient medicine” (1). In his investigative research, he indicates that S.aureus “accounts for 76% of culturable skin and soft-tissue infections, of which 59% are MRSA” (1). S.aureus’ ability to resist antibiotics such as methicillin, really makes it a threat if left unattended or treated, it can often lead to threatening situations and in many cases, death. CONCLUSION • Unknown #15 was identified as a gram positive, coccus shaped bacterium. • It tested catalase positive and oxidase negative. • Further testing revealed that Unknown #15 is an osmotolerant bacterium. • It is also a mesophile and an alkaliphile. • It is beta hemolytic, sensitive to Novobiocin, and can ferment mannitol. • It also has the ability to coagulate plasma. • Therefore, unknown bacterium #15 was identified as staphylococcus aureus. REFERENCES Gregory J. Moran, Anusha Krishnadasan, Rachel J. Gorwitz, Gregory E. Fosheim, Linda K. McDougal, Roberta B. Carey, David A. Talan . Methicillin-Resistant S. aureusInfections among Patients in the Emergency Department. New England Journal of Medicine, Volume 355, Number 7 (August 2006), pp. 666-674, <http://0ejournals.ebsco.com.opac.sfsu.edu/direct.asp?ArticleID=41418A227A8D84FC11E4>.