1 Yeast Are People Too: sourdough fermentation from the microbe's
advertisement

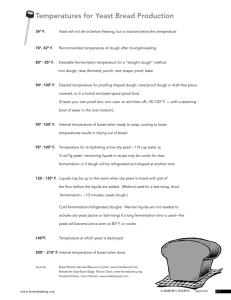
Yeast Are People Too: sourdough fermentation from the microbe’s point of view Jessica A. Lee An unbaked loaf of sourdough bread is a garden, home to microorganisms of diverse species and functions. It is an intimate working relationship between microbes and humans; it is also a potent reminder of the culinary benefits of biodiversity. Fermentation is primarily the business of yeast and bacteria. We humans aren’t actually very good at it; we cultivate microbes to do the biochemical work for us. So while fermentation is often seen as a craft, it may in fact more accurately be described a sort of micro-agriculture. The Botany of Desire: a plant’s eye view of the world (2001), a book that Michael Pollan wrote with the purpose of bringing attention to the lives and needs of the plants we eat. In his book, Pollan illustrates that agriculture is not simply a practice in which humans manipulate plants to express the traits we find desirable, but in which plants evolve traits that coerce humans to aid in the survival of their species... and also in which the traits of plants help to shape human history. In a similar fashion, humans and our food are changed by the microbes we work with, just as much as those microbes are changed by us. We have done this breadmaking thing, harnessing the microbes we find around us, for as long as history has been recorded. However, it is only in the last few decades that we have developed the techniques to be able to understand in greater chemical and biological detail what exactly happens in that bread dough, and to use that knowledge to improve bread further. Here, I will give an introduction to that knowledge, with the hopes of encouraging bakers to think like microbes. Breadmaking is the work of microorganisms. Unlike cookies, cakes, and quickbreads, yeast-leavened bread is a baked good that requires the participation of biology in the kitchen. And sourdough bread is defined by that biology. The word ‘sourdough’ is sometimes used to refer to bread leavened with ‘wild-caught’ yeast rather than the commercially-cultivated Saccharomyces cerevisiae; or sometimes to the distinctive profile of flavor, texture, and keeping qualities associated with bread made by sourdough fermentation. However, here we use sourdough to refer specifically to the community of microorganisms behind that fermentation: a diverse consortium of yeasts and lactic acid-producing bacteria. Before the invention of the term ‘sourdough,’ it was the only method by which bread was made, for most of history, before the advent of commercial bakers’ yeast production. Bread has always been leavened with yeasts captured from the environment, and bacteria were often captured at the same time. Moreover, sourdough yeast often fare better when living alongside bacteria. However, sourdough bread is now a novelty rather than the default method of production; the default is now bread made with commercial yeast is grown as a monoculture - a single species - and no significant bacterial activity. The 1 popularity of ‘conventional’ bread is partly due to the simplicity and regularity of the process, and partly because of the wide appeal of the mild flavor and very regular texture achievable without bacterial activity. Sourdough has therefore earned itself a niche in small-scale artisanal baking for other qualities: complex flavor, rugged texture, and good shelf life. All of those special qualities of sourdough bread may be attributed to the complex communities of yeast and bacteria that build it. And here is the place to remind the reader that sourdough microorganisms do not improve sourdough bread out altruism; they are interested in nothing more than their own survival and reproduction. It just so happens that many of the processes they carry out for survival contribute to an excellent artisanal product. The making of a good sourdough is therefore a symbiosis between baker and microbes, in which both win. Colonization: what starts a starter? The diverse assemblage of yeast and bacteria that leaven sourdough bread first begins its relationship with the baker when the organisms colonize the flour-water batter that will become the starter. The mixture is allowed to sit for hours or days until microbial activity is observed, in the form of bubble formation, sour or alcoholic smell, and thinner texture (signs of microbial activity, the specifics of which are described below). This formation of a ‘spontaneous sourdough’ is actually one of ecological succession Where did the bacteria and yeast come from? Everything is everywhere, and the environment selects. This is known as the Baas-Becking hypothesis, after the Dutch microbiologist who used the words to describe his observation that all microorganisms are ubiquitous but that the characteristics of any particular environment determine which ones succeed at being most abundant there. Bacteria and yeast are in the air; on the bowls and spoons used to mix the batter; in the flour itself; in ingredients such as fruit or yogurt added to help kick-start the process. Then, of all of the microbes that find their way into the burgeoning starter, only a few kinds are able to survive in the world of the flour-water paste. The initial several hours after inoculation see rapid changes in the microbial community. But once an active sourdough starter has been established, the community of microorganisms remains quite stable in abundance and composition 1. It consists primarily of one or a few species of yeast, and up to several species of bacteria, almost exclusively lactic acid bacteria. Table 1 lists common sourdough microorganisms; no one sourdough contains all of these species, but the many species show up in sourdoughs 1 Peter Stolz, ‘Biological Fundamentals of Yeast and Lactobacilli Fermentation in Bread Dough’, in Handbook of Dough Fermentations (New York, NY: Marcel Dekker, Inc., 2003), pp. 23-42. 2 from around the world. Once the population has reached stability, microbial counts tend to range 107 – 109 live active bacterial cells per gram of dough, and 102 – 107 yeast cells 2. The baker may exert some control over the factors that determine what survives: the kind of food available (that is, variety of flour and/or added sweeteners); the mechanisms of breathing that are possible (how well the batter is aerated); the temperature; how much starvation the organisms must endure (the fermentation time before dough refreshment); and the physical nature of the substrate (the hydration of the dough) 3. In the end, by setting his fermentation parameters and then attracting all the potential participants he can, the baker will automatically end up with the population that is best adapted to survive in his particular dough. Importantly, the baker’s choice of fermentation parameters is not the only force that controls these factors. All the organisms in the starter are simultaneously struggling to survive, and at the same time, they too are shaping their environment in ways that make it more or less hospitable to other organisms. There is evidence that certain combinations of yeast and bacteria species are more likely to coexist than others; however, results from different studies sometimes disagree about the associations they observe 4. One explanation for these conflicts is the confusing nature of microbial taxonomy and naming; even within one species, sub-species (strains) often show such great metabolic diversity that it is easy to believe that when one study describes the behavior of L. plantarum, it may well be talking about a very different L. plantarum than another study 5. In opposition to the Baas-Becking hypothesis is the argument that biogeography may play a role—perhaps not everything is everywhere, and location does matter. Certainly, the importance of environmental selection on narrowing down a sourdough’s inhabitants is clear: only a handful of genera of yeast and bacteria have ever been found in any sourdoughs anywhere (evident in Table 1). However, within those genera, the role of selection is less clear, and there is growing evidence that in fact what determines whether L. plantarum or L. sanfranciscensis flourishes in a certain sourdough is simply who got there first: Scheirlinck and colleagues, in their survey of traditional Belgian sourdoughs, found the sourdough microbial community to be more dependent on bakery environment than on dough composition 6. This indicates that regardless of dough makeup and fermentation conditions, a baker may have even more control over the population of his sourdough by his choice of inoculum. 2 Sonya Siragusa and others, ‘Taxonomic Structure and Monitoring of the Dominant Lactic Acid Bacteria Population during Wheat Flour Sourdough Type I Propagation by using Lactobacillus sanfranciscensis Starters’, Appl. Environ. Microbiol., 2008.. 3 Stolz, pp. 23-42. 4 Ilse Scheirlinck and others, ‘Taxonomic Structure and Stability of the Bacterial Community in Belgian Sourdough Ecosystems as Assessed by Culture and Population Fingerprinting’, Appl. Environ. Microbiol., 74 (2008), 2414-2423. 5 Olimpia Pepe and others, ‘Technological and Molecular Diversity of Lactobacillus plantarum Strains Isolated from Naturally Fermented Sourdoughs’, Systematic and Applied Microbiology, 27 (2004), 443-453. 6 Ilse Scheirlinck and others, ‘Influence of Geographical Origin and Flour Type on Diversity of Lactic Acid Bacteria in Traditional Belgian Sourdoughs’, Appl. Environ. Microbiol., 73 (2007), 6262-6269. 3 In any stable sourdough starter, regardless of the species makeup of the microbial community, you will find the same basic processes always being carried out by someone or other. In the sections that follow, we discuss the daily business that goes on in a sourdough community. A day in the life of a sourdough Upon being born in a sourdough, a microbe finds itself in a world of carbohydrates, proteins, fats, and water. The same basic ingredients are present in a liquid starter or in a solid bread dough; Figure 1 presents an image of the maze that is the solid dough, a network of proteins with suspended starch grains. A microbe’s first line of business is to eat, in order to generate energy. Like humans, yeast and lactobacilli get their energy from carbohydrates... eating, in fact, a very small portion of the very bread dough they live in, and simultaneously excreting their waste into it, before we consume it. As unappetizing as this sounds, the changes that microbes work on sourdough bread are for the better - they break down molecules to change the texture and digestibility of the bread, and create new molecules to change its taste and keeping properties. The main source of carbohydrates in bread dough is starch, which makes up approximately 70 per cent of wheat flour by weight 7. Starch is composed of long chains of glucose molecules strung together into molecules called amylose and amylopectin, protected inside starch granules. Upon milling the granules are damaged and starch becomes physically accessible to consumption, but the long chains remain chemically unusable to yeast and bacteria until they are broken down into smaller fragments. This job is done by the enzymes α- and β-amylase, which chop the bonds between the monomers to release smaller sugars, primarily the single sugar glucose and its disaccharide counterpart, maltose. (Figure 2) Remarkably, most of the breakdown of starch is done by enzymes present in the wheat grain itself. A wheat grain carries amylases in preparation for the day it will germinate, when it will need to break down the stored starch into glucose to give the growing plant quick energy. Of course, a wheat grain that has been milled into flour will never germinate, but the enzymes are still there and active in the bread dough. The fate of carbohydrates: yeast fermentation Yeast have two ways of eating. Like animals, they can use oxygen to turn glucose into carbon dioxide gas. This process is the most efficient way of obtaining energy from glucose, so when given oxygen, yeast will do this first. For every molecule of glucose, six molecules of oxygen are consumed and six molecules each of carbon dioxide and water are produced: C6H12O6 + 6 O2 → 6 CO2 + 6 H2O 7 Harold McGee, On Food and Cooking: the Science and Lore of the Kitchen (New York, NY: Scribner, 2004). 4 1 glucose + 6 oxygen → 6 carbon dioxide + 6 water However, in the absence of oxygen, they can still obtain energy from sugar by a less efficient method: fermentation. Fermentation is a general term for several ways of metabolizing sugar without oxygen. Yeast carry out ethanol fermentation: they turn one molecule of glucose into two molecules of ethanol and two molecules of carbon dioxide: C6H12O6 → 2 C2H5OH + 2 CO2 1 glucose → 2 ethanol + 2 carbon dioxide Without oxygen, yeast are unable to break apart all of the C atoms, leaving quite a bit of chemical energy in ethanol. Because of the energy yield, yeast are best at reproducing when they have oxygen to breathe with; therefore, in the commercial production of bakers’ yeast for sale, yeast cultures are kept well-aerated. Also evident from the formulas above, yeast are best at producing gas when they are given oxygen, so a well-aerated dough becomes a wellleavened one. Conversely, yeast will not produce alcohol in the presence of oxygen; therefore, the highest yield possible of alcohol is generated by an anaerobic culture. To further encourage efficiency of fermentation in bread dough, the most important conditions for the baker to control are temperature and the content of sugar and salt. In general, all microbes eat, breathe, and grow faster at higher temperatures. The optimal fermentation temperature for most commercial yeasts is 38°C, but sourdough yeasts may vary widely in their favorite conditions 8. Sugar and salt slow down fermentation: while a little sugar is exactly what yeast love to eat and can stimulate rapid growth, high concentrations of sugar in the dough create strong osmotic pressure, threatening to draw water out of both yeast and bacterial cells. (This is the same force at work in pickles and jams; when there is a higher sugar and salt concentration outside the cell than inside the cell, water will move out of the cell, leaving a shriveled, desiccated bacterium.) While bacteria and yeast can fight osmotic pressure to some extent, and some sourdoughassociated microbes are hardier than commercial yeast, the stress of doing so slows down their fermentative activity. For this reason, very sweet doughs require more yeast and/or longer fermentation times than lean doughs. Happily, as microbes consume the sugar around them they also lower the osmotic pressure they must fight 9. In a conventional bread dough, at its fastest, a gram of commercial baker’s yeast can ferment 0.3-0.7 g carbohydrates per hour. In the entire course of fermentation, the sugar consumed by the yeast is equivalent to about 3per cent of the total flour weight. That means that in a 1-lb loaf, approximately 5 g of carbon dioxide is produced, equivalent to 1500 cm3 (more than ½ gallon) of gas. Most of this remains dissolved in the dough or in small bubbles - dependent on the structure of the dough - until baking. An equal amount of sugar is converted to ethanol at the same time 10. 8 Daniel H. Maloney and James J. Foy, ‘Yeast Fermentations’, in Handbook of Dough Fermentations (New York, NY: Marcel Dekker, Inc., 2003), pp. 43-62. 9 Maloney and Foy, pp. 43-62. 10 Maloney and Foy, pp. 43-62. 5 Sourdough yeasts are almost certainly less efficient than standard bakery yeasts, as they have not been bred for optimum efficiency in conversion of sugar to CO2 or ethanol. Instead, they compromise efficiency for other fitness advantages, such as acid tolerance or the ability to consume a diverse range of energy sources. The fate of carbohydrates: bacterial fermentation All of the above processes occur in every bread yeast-raised bread, both commercial and sourdough. However, as mentioned above, the distinguishing characteristic of sourdough is the population of lactic acid bacteria it harbors alongside the yeast. Unlike yeast, lactic acid bacteria cannot use oxygen to break glucose all the way down to carbon dioxide; they make their living only by fermentation. But instead of producing ethanol, they produce, as their name suggests, lactic acid. C6H12O6 → 2 CH3CHOHCOOH 1 glucose --> 2 lactic acid Bacteria that produce only lactic acid are classified as homofermentative. Heterofermentative bacteria produce both lactic acid and either acetic acid or ethanol: C6H12O6 → CH3CHOHCOOH + C2H5OH + CO2 1 glucose --> 1 lactic acid + 1 ethanol + 1 carbon dioxide or C6H12O6 + O2→ CH3CHOHCOOH + CH3COOH + CO2 + H2O 1 glucose + 1 oxygen --> 1 lactic acid + 1 acetic acid + 1 carbon dioxide + 1 water The concentration of oxygen present determines whether more ethanol or more acetic acid is produced 11 12. Both homo-fermentative and hetero-fermentative bacteria may be found in sourdough bread. The fact that both yeast and bacteria like to eat glucose would seem to be a perfect set-up for a competitive relationship, rather than the symbiosis that we recognize as typical of sourdough. The answer lies in the fact that, as mentioned before, the glucose in the above equations is actually not very abundant in bread dough, but rather it is the product of substantial work that the microbes must put into breaking down larger molecules, and subsequently importing them into the cell interior. Carbohydrate degradation and transport is one of the main differentiating features among microbial species. Each species of microorganism has the capability to process a different suite of carbohydrates to obtain the glucose it ultimately eats, so different species often do not compete for the same substrate. However, because glucose is such a quick and easy food, when it is abundant many organisms will shut down their ability to use other substrates so that they can concentrate on consume as much glucose as possible—a behavior known as ‘glucose repression’ 12. 11 12 Otto Kandler, ‘Carbohydrate metabolism in lactic acid bacteria’, Antonie van Leeuwenhoek, 49 (1983), 209-224. Maloney and Foy, pp. 43-62. 6 Typical San Francisco sourdough provides just one example of the interlocking metabolic relationships in yeast and bacterial consortia. Many yeast, including the S. cerevisiae sold commercially, are able to recognize the disaccharide maltose and transport it into the cell, where it is then broken down into two molecules of glucose and used for energy. However, S. exiguus, a species of yeast found commonly in San Francisco sourdough, cannot. At the same time, L. sanfranciscensis, one of the lactic acid bacteria most commonly found in sourdough, can use only maltose or glucose. When it takes maltose into the cell it often turns it into glucose faster than it can use it up. As a result, it excretes glucose back into the medium, where it is perfect fodder for the yeast there 13 14. It may well be that S. exiguus simply lost the ability to uptake maltose because it had no need to, and in fact is better off not competing with the bacteria with whom it coexists. At the same time, the high concentration of glucose in the medium triggers glucose repression in other organisms, so that they lose their ability to consume maltose. Notably, L. sanfranciscensis is not subject to glucose repression, and so is left to consume maltose, free of competition 15. An acid environment Every time a sourdough bacterium eats, it produces acid. Although acid appears to be simply a byproduct of the way bacteria obtain energy, it also constitutes one of the most important chemical characteristics of the sourdough environment. To a sourdough microbial community, acidity acts as a powerful weapon to keep other organisms at bay. The pH in a sourdough starter can fall as low as 4 or lower, and sourdough bacteria are tolerant of acidic environments but many other bacteria are not. Once bacteria start producing acid, they quickly clear the field of competitors so that they can continue to reproduce and to produce more acid. So while the single cell sees acid production as having only to do with energy production, from the perspective of the population it also serves the purpose of self-defense, and confers such an evolutionary advantage that it would be unfair to call it just an accidental byproduct of metabolism. And while acid production is obviously selfish to the bacteria in a multi-faceted fashion, it is also extremely useful to the yeast it lives with, by making the environment hospitable only to a few certain kinds of yeast. Sourdough yeast such as S. exiguus are extremely tolerant of acidity and flourish in the low-pH environment of the sourdough starter, whereas S. cerevisiae are not 16. The fate of proteins 13 H Neubauer and others, ‘Mechanism of maltose uptake and glucose excretion in Lactobacillus sanfrancisco.’, Journal of Bacteriology, 176 (1994), 3007-3012. 14 Peter Stolz and others, ‘Utilisation of maltose and glucose by lactobacilli isolated from sourdough’, FEMS Microbiology Letters, 109 (1993), 237-242. 15 Stolz and others, 237-242. 16 M. Antonia Martinez-Anaya, ‘Associations and interactions of Microorganisms in Dough Fermentations: Effects on Dough and Bread Characteristics’, in Handbook of Dough Fermentations (New York, NY: Marcel Dekker, Inc., 2003), pp. 63-95. 7 Like humans, bacteria and yeast not only need to eat carbohydrates to get energy; they also need to consume proteins with which to build new cells. And as with sugars, microbes generally do not consume proteins whole, but need them broken down into their building blocks (amino acids). About 10per cent of wheat flour is composed of the long protein chains glutenins and gliadins. To the wheat grain, they are a way of storing protein for the future baby plant; in the bread dough, they interact to form the elastic gluten matrix that traps gas and enables bread to rise. Lactic acid bacteria possess proteases—enzymes to break down proteins into their constituent amino acids 17. This means that as microbes do their job mining amino acids from the dough they are in fact gradually tearing down the walls around them bit by bit. And their activity is strikingly evident in the changes in the texture (rheological properties) of the dough after fermentation: sourdoughs are measurably softer than doughs fermented only with yeast 18. To the baker or bread consumer, these changes can be construed as either positive or negative, depending on the desired texture; to the microorganism, the main benefit is nutritional. Studies investigating the role of sourdough in bread dough rheology have revealed that, in fact, bacterial enzymes are not responsible for most of the protein breakdown that occurs during sourdough fermentation 19. But sourdough bacteria are in fact responsible for protein breakdown in another, indirect way: once again, it is a function of their acidproducing behavior. Just as wheat grains contain enzymes to break down starch and release sugar upon germination, they also contain enzymes to break down gluten and release smaller peptides for the growing baby plant to use 20. Importantly, they are at their most active at low pH (around 4.5) 21. Though rarely seen in conventional bread dough, this is just the pH that lactic acid bacteria create in sourdough. So here is yet another way in which bacterially produced acids - ostensibly just a waste product from their method of getting energy - are in fact essential to their survival: without those acids, they would not be nearly so successful at getting the protein they need. And, of course, this particular evolutionary advantage - exceptional protein procurement through acid production - only plays out in the environment of the bread dough. In dairy or meat products, such acid-loving plant proteases are not present, so, unable to manipulate the enzymes of others, bacteria must make their own 22. As mentioned before, proteolysis in sourdough bread affects dough rheology ... but human interest in this proteolysis goes beyond that of bread dough quality, to that of 17 C.L. Gerez, G.C. Rollan and G.F. Valdez, ‘Gluten breakdown by lactobacilli and pediococci strains isolated from sourdough’, Letters in Applied Microbiology, 42 (2006), 459-464. 18 Martinez-Anaya, pp. 63-95. 19 C. Thiele, S. Grassl and M. Ganzle, ‘Gluten Hydrolysis and Depolymerization during Sourdough Fermentation’, Journal of Agricultural and Food Chemistry, 52 (2004), 1307-1314. 20 Andrea Bottari and others, ‘Major proteinase hydrolysing gliadin during wheat germination’, Phytochemistry, 43 (1996), 39-44. 21 Mikhail A. Belozersky, Sh. T. Sarbakanova and Ya. E. Dunaevsky, ‘Aspartic proteinase from wheat seeds: isolation, properties and action on gliadin’, Planta, 177 (1989), 321-326. 22 Jeffrey E. Christensen and others, ‘Peptidases and amino acid catabolism in lactic acid bacteria’, Antonie van Leeuwenhoek, 76 (1999), 217-246. 8 human health. The disease of greatest concern to potential bread-eaters is probably celiac disease, an autoimmune disorder in which the products of digestion of gluten - the very wheat protein we have just been discussing - cause an allergic reaction at the stomach lining and unpleasant symptoms including abdominal discomfort and malabsorption 23. Today, there is still only one effective treatment: lifelong avoidance of all glutencontaining foods. However, interest has arisen in the proteolytic properties of sourdough bread. Preliminary investigations have found that some sourdough breads can be tolerated by celiac patients 24; that culturing wheat flour with a combination of extracted fungal proteases and live sourdough bacteria certain can make the toxic peptides all but disappear; and that both bread and pasta of decent culinary quality can be made from treated flour 25 26. Further investigations are necessary, however, to bring the process to commercial viability. Making flavor There is no end to the list of important functions that dough acidification plays in sourdough bread. Not the least, of course, is the sour flavor from which sourdough gets its name. This flavor is also dependent on the environment the bacteria live in; it is determined by the balance between lactic and acetic acids - a higher concentration of acetic relative to lactic acid provides a sharper flavor, and, as explained above, bacteria produce more acetic acid when provided with more oxygen. Stepping away from the acid question temporarily, another remarkable talent of sourdough microorganisms is the production of many other complex flavor compounds; see Table 2 for a brief list of some of the compounds and their associated flavors. Most of these molecules are simply ‘secondary metabolites’ - byproducts of sundry metabolic activities, especially the processing of amino acids 27. Many of these flavoring compounds remain in the final bread; in addition, the baking process contributes greater flavor to the crust through Maillard reactions - the heat-induced combinations of sugars and proteins 28. In general, the greater the microbial diversity in a sourdough, the more different processes that can occur, and therefore the more complex the flavor profile 29; as Table 2 shows, some flavor compounds are detected only in doughs with both yeast and bacteria. In addition, longer fermentation times allow for microbes to produce more secondary 23 Antonio Di Sabatino and Gino Roberto Corazza, ‘Coeliac disease’, The Lancet, 373 (2009), 1480-1493. Raffaella Di Cagno and others, ‘Sourdough Bread Made from Wheat and Nontoxic Flours and Started with Selected Lactobacilli Is Tolerated in Celiac Sprue Patients’, Appl. Environ. Microbiol., 70 (2004), 1088-1096. 25 C.G. Rizzello and others, ‘Highly Efficient Gluten Degradation by Lactobacilli and Fungal Proteases during Food Processing: New Perspectives for Celiac Disease’, Appl. Environ. Microbiol., 73 (2007), 4499-4507. 26 Maria De Angelis and others, ‘Mechanism of Degradation of Immunogenic Gluten Epitopes from Triticum turgidum L. var. durum by Sourdough Lactobacilli and Fungal Proteases’, Appl. Environ. Microbiol., 76 (2010), 508-518. 27 Maloney and Foy, pp. 43-62. 28 McGee. 29 Luc De Vuyst and others, ‘The Biodiversity of Lactic Acid Bacteria in Greek Traditional Wheat Sourdoughs Is Reflected in Both Composition and Metabolite Formation’, Appl. Environ. Microbiol., 68 (2002), 6059-6069. 24 9 metabolites. These are both reasons that conventional bread, made with only S. cerevisae and short fermentation times, has a much simpler flavor profile than traditional sourdough breads do. However, there is no reason that S. cerevisiae and short fermentation times must necessarily go hand-in-hand, and both mild-flavored, shortfermented sourdoughs as well as more complex-flavored, long-fermented conventional yeast breads are well within the realm of possibility. Fighting spoilage That sourdough bread spoils more slowly than conventional bread has long been an accepted fact. Of course, there is significant interest in the mechanisms behind sourdough keeping properties, and in the possibility of harnessing those mechanisms to improve any bread even without the full sourdough process. The main culprits of bread spoilage targeted by scientific study are the Bacillus species, several of which are blamed with causing ‘ropiness’ in baked bread: ‘unpleasant fruity odor, followed by enzymatic degradation of the crumb that becomes soft and sticky because of the production of extracellular slimy polysaccharides’ 30. We have already discussed the self-defense strategies of sourdough bacteria that allow them to dominate the living bread dough community; the inhibition of Bacillus invasion in baked bread, after the native lactic acid bacteria have been cooked to death, is another issue. However, studies indicate that the two main avenues of sourdough bacteria selfdefense do live on through the baking process and are likely the cause for the bread’s keeping properties. These avenues are acidity and antibiotic compounds. The mechanism of acidity production is the same that has been discussed previously. In addition, many lactic acid bacteria - especially L. plantarum, L. bavaricus, L. curvatus have been found to be capable of inhibiting Bacillus growth by the production of antibiotic compounds that they excrete - small, protein-based molecules called bacteriocins. Many of these bacteriocins are heat-resistant and thus probably survive baking 31 32 33. The choice of conventional yeast bread over sourdough for flavor or ease of production requires a compromise in keeping qualities. Chemical preservatives are one solution-sodium propionate and calcium propionate are favorite antifungal agents that have been proven effective against rope spoilage with little impact on the activity of yeast (which is also a fungus) 34. However, growing consumer demand for ‘all-natural’ foods has prompted greater interest in the use of compounds derived from sourdough bacteria, and 30 Olimpia Pepe and others, ‘Rope-Producing Strains of Bacillus spp. from Wheat Bread and Strategy for Their Control by Lactic Acid Bacteria’, Appl. Environ. Microbiol., 69 (2003), 2321-2329. 31 A. Corsetti, L. Settanni and D. Van Sinderen, ‘Characterization of bacteriocin-like inhibitory substances (BLIS) from sourdough lactic acid bacteria and evaluation of their in vitro and in situ activity’, Journal of Applied Microbiology, 96 (2004), 521-534. 32 Luc De Vuyst and Frédéric Leroy, ‘Bacteriocins from Lactic Acid Bacteria: Production, Purification, and Food Applications’, Journal of Molecular Microbiology and Biotechnology, 13 (2007), 194-199. 33 Paola Lavermicocca and others, ‘Purification and Characterization of Novel Antifungal Compounds from the Sourdough Lactobacillus plantarum Strain 21B’, Appl. Environ. Microbiol., 66 (2000), 4084-4090. 34 Sharon Gerdes, ‘Keeping molds, bacteria at bay’, Baking Management, 2004 <http://bakingmanagement.com/ingredients/bm_imp_7448/> [accessed 3 May 2010]. 10 the possibility of isolating the compounds for use even in bread fermented without lactic acid bacteria. Although many potential anti-rope bacteriocins have been identified in the laboratory, only a few have been proven to work in bread; among these is nisin, produced by Lactococcus lactis (common in dairy products), which kills other bacteria by poking holes in their cell membranes 35. The commercial success of nisin raises hopes for future developments with other bacteriocins. The search for novel biological anti-spoilage agents among sourdough microbes is not unlike the search for biological pesticides among soil-associated bacteria that produced Bacillus thuringiensis as an extremely popular insecticide, or even the search among rainforest plants for the next million-dollar drug. Sourdough microbiota are an equally valuable repository of genetic and biochemical diversity, and the remarkable properties of the bread they produce may be interpreted as a sensual reminder of the value of biodiversity in the food we eat. Sourdough microbes and humans have led a symbiotic existence for millenia, in which humans have created an environment in which the microorganisms may thrive, and the microorganisms have in turn worked to turn their living environment into bread rich in flavor, toothsome in texture, free of pathogens, and slow to spoil. It is only natural that interest in traditional sourdough baking is re-emerging in conjunction with interest in traditional forms of sustainable agriculture and the resurrection of heirloom crops and animals; sourdough microorganisms are equally important teammates in food production. Table 1. Microorganisms commonly found in sourdough cultures. Data from Scheirlinck et al. (2007); Maloney and Foy (2003); Stolz (2003). Yeast Candida boldinii Candida guilliermondii Candida holmii Candida krusei/ crusei Candida milleri Candida stellata Candida tropicalis Hansenula anomala Hansenula subpelliculosa Hansenula tropicalis Pichia polymorpha Pichia saitoi Saccharomyces cerevisae Saccharomyces dairensis Bacteria Enteroccocus mundtii Lactobacillus acidophilus Lactobacillus amylovorus Lactobacillus brevis Lactobacillus buchneri Lactobacillus casei Lactobacillus casei Lactobacillus confusus Lactobacillus crispatus Lactobacillus crustorum Lactobacillus curvatus Lactobacillus delbrueckii Lactobacillus farciminis Lactobacillus fermentum 35 J. Lubelski and others, ‘Biosynthesis, immunity, regulation, mode of action and engineering of the model lantibiotic nisin’, Cellular and Molecular Life Sciences, 65 (2008), 455-476. 11 Lactobacillus Saccharomyces ellipsoideus fructivorans Saccharomyces exiguus Lactobacillus hammesii Saccharomyces fructuum Lactobacillus helveticus Saccharomyces inusitatus Lactobacillus johnsonii Lactobacillus Torulopsis holmii namurensis Saccharomyces chevalieri Lactobacillus nantensis Lactobacillus Saccharomyces curvatus parabuchneri Saccharomyces inusitatus Lactobacillus paracasei Saccharmoyces panis Lactobacillus fermentati paralimentarius Candida norvegensis Lactobacillus plantarum Lactobacillus pontis Lactobacillus reuteri Lactobacillus rossiae Lactobacillus sakei Lactobacillus sanfranciscensis Lactobacillus spicheri Leuconostoc mesenteroides Pediococcus acidilactici Pediococcus pentosaeceus Weissella confusa Weissella cibaria b) a) Figure 1. a) Scanning electron microscopy image, 12 optimally kneaded dough. b) Scanning electron microscopy, highly overkneaded dough. From Belitz, Grosch and Schieberle (2009) a) c) b) Figure 2. Chemical structures of a) glucose; b) maltose; c) amylose; d) amylopectin. d) Table 2. Flavoring compounds detected in sourdoughs. Compounds produced by yeast; either yeast or bacteria; or only in the presence of both. Data from Maloney and Foy (2003). Compound alcohols ethanol n-propranol n-pentanol ( amyl alcohol) n-hexanol carbonyls acetaldehyde propionic acid n-butyric acid i-butyric acid n-valeric acid hexanoic acid acetone methylpropanal 2-methyl-1-butanal 3-methyl butanal 2,3-butanedione (diacetyl) 3-hydroxy-2-butanone produced by bacteria or Flavor yeast alcoholic either fusel-like, burning either fusel-like, burning yeast alcoholic either pungent yeast rancid either rancid butter either sweaty either rancid butter either unpleasant, copralike yeast aromatic, sweet either malty yeast malty yeast malty yeast butter yeast butter only both 13 esters (acetoin) n-hexanal trans-2-heptanal methional ethyl acetate 2-acetyl-1-pyrroline i-amyl acetate phenethyl acetate 2,3-methylbutyl acetate n-hexyl acetate ethyl n-propanoate ethyl n-hexanoate fruity green, fatty malty ether, pineapple roasty fruity fruity apple peel, banana pear, bittersweet rum, pineapple pineapple, banana either either yeast either yeast yeast yeast yeast only both only both either Bibliography Belozersky, Mikhail A., Sh. T. Sarbakanova, and Ya. E. Dunaevsky, ‘Aspartic proteinase from wheat seeds: isolation, properties and action on gliadin’, Planta, 177 (1989), 321326. Bottari, Andrea, Antonella Capocchi, Debora Fontanini, and Luciano Galleschi, ‘Major proteinase hydrolysing gliadin during wheat germination’, Phytochemistry, 43 (1996), 39-44 . Christensen, Jeffrey E., Edward G. Dudley, Jeffrey A. Pederson, and James L. Steele, ‘Peptidases and amino acid catabolism in lactic acid bacteria’, Antonie van Leeuwenhoek, 76 (1999), 217-246 . Corsetti, A., L. Settanni, and D. Van Sinderen, ‘Characterization of bacteriocin-like inhibitory substances (BLIS) from sourdough lactic acid bacteria and evaluation of their in vitro and in situ activity’, Journal of Applied Microbiology, 96 (2004), 521-534. De Angelis, Maria, Angela Cassone, Carlo G. Rizzello, Francesca Gagliardi, Fabio Minervini, Maria Calasso, and others, ‘Mechanism of Degradation of Immunogenic Gluten Epitopes from Triticum turgidum L. var. durum by Sourdough Lactobacilli and Fungal Proteases’, Appl. Environ. Microbiol., 76 (2010), 508-518. De Vuyst, Luc, and Frédéric Leroy, ‘Bacteriocins from Lactic Acid Bacteria: Production, Purification, and Food Applications’, Journal of Molecular Microbiology and Biotechnology, 13 (2007), 194-199. De Vuyst, Luc, Vincent Schrijvers, Spiros Paramithiotis, Bart Hoste, Marc Vancanneyt, Jean Swings, and others, ‘The Biodiversity of Lactic Acid Bacteria in Greek Traditional Wheat Sourdoughs Is Reflected in Both Composition and Metabolite Formation’, Appl. Environ. Microbiol., 68 (2002), 6059-6069. 14 Di Cagno, Raffaella, Maria De Angelis, Salvatore Auricchio, Luigi Greco, Charmaine Clarke, Massimo De Vincenzi, and others, ‘Sourdough Bread Made from Wheat and Nontoxic Flours and Started with Selected Lactobacilli Is Tolerated in Celiac Sprue Patients’, Appl. Environ. Microbiol., 70 (2004), 1088-1096. Di Sabatino, Antonio, and Gino Roberto Corazza, ‘Coeliac disease’, The Lancet, 373 (2009), 1480-1493. Gerdes, Sharon, ‘Keeping molds, bacteria at bay’, Baking Management, 2004 <http://baking-management.com/ingredients/bm_imp_7448/> [accessed 3 May 2010]. Gerez, C.L., G.C. Rollan, and G.F. Valdez, ‘Gluten breakdown by lactobacilli and pediococci strains isolated from sourdough’, Letters in Applied Microbiology, 42 (2006), 459-464. Kandler, Otto, ‘Carbohydrate metabolism in lactic acid bacteria’, Antonie van Leeuwenhoek, 49 (1983), 209-224. Lavermicocca, Paola, Francesca Valerio, Antonio Evidente, Silvia Lazzaroni, Aldo Corsetti, and Marco Gobbetti, ‘Purification and Characterization of Novel Antifungal Compounds from the Sourdough Lactobacillus plantarum Strain 21B’, Appl. Environ. Microbiol., 66 (2000), 4084-4090. Lubelski, J., R. Rink, R. Khusainov, G. Moll, and O. Kuipers, ‘Biosynthesis, immunity, regulation, mode of action and engineering of the model lantibiotic nisin’, Cellular and Molecular Life Sciences, 65 (2008), 455-476. Maloney, Daniel H., and James J. Foy, ‘Yeast Fermentations’, in Handbook of Dough Fermentations (New York, NY: Marcel Dekker, Inc., 2003), pp. 43-62. Martinez-Anaya, M. Antonia, ‘Associations and interactions of Microorganisms in Dough Fermentations: Effects on Dough and Bread Characteristics’, in Handbook of Dough Fermentations (New York, NY: Marcel Dekker, Inc., 2003), pp. 63-95. McGee, Harold, On Food and Cooking: the Science and Lore of the Kitchen (New York, NY: Scribner, 2004). Neubauer, H, E Glaasker, W P Hammes, B Poolman, and W N Konings, ‘Mechanism of maltose uptake and glucose excretion in Lactobacillus sanfrancisco.’, Journal of Bacteriology, 176 (1994), 3007-3012. Ng, Henry, ‘Factors Affecting Organic Acid Production by Sourdough (San Francisco) Bacteria’, Appl. Environ. Microbiol., 23 (1972), 1153-1159. Pepe, Olimpia, Giuseppe Blaiotta, Giancarlo Moschetti, Teresa Greco, and Francesco 15 Villani, ‘Rope-Producing Strains of Bacillus spp. from Wheat Bread and Strategy for Their Control by Lactic Acid Bacteria’, Appl. Environ. Microbiol., 69 (2003), 2321-2329. Pollan, Michael. The Botany of Desire: A Plant’s Eye View of the World. New York, NY: Random House, 2001. Pepe, Olimpia, Guiseppe Blajotta, Marilena Anastasio, Giancarlo Moschetti, Danilo Ercolini, and Francesco Villani, ‘Technological and Molecular Diversity of Lactobacillus plantarum Strains Isolated from Naturally Fermented Sourdoughs’, Systematic and Applied Microbiology, 27 (2004), 443-453. Rizzello, C.G., M. De Angelis, R. Di Cagno, A. Camarca, M. Silano, I. Losito, and others, ‘Highly Efficient Gluten Degradation by Lactobacilli and Fungal Proteases during Food Processing: New Perspectives for Celiac Disease’, Appl. Environ. Microbiol., 73 (2007), 4499-4507. Scheirlinck, Ilse, Roel Van der Meulen, Ann Van Schoor, Marc Vancanneyt, Luc De Vuyst, Peter Vandamme, and others, ‘Influence of Geographical Origin and Flour Type on Diversity of Lactic Acid Bacteria in Traditional Belgian Sourdoughs’, Appl. Environ. Microbiol., 73 (2007), 6262-6269. ---, ‘Taxonomic Structure and Stability of the Bacterial Community in Belgian Sourdough Ecosystems as Assessed by Culture and Population Fingerprinting’, Appl. Environ. Microbiol., 74 (2008), 2414-2423. Siragusa, Sonya, Raffaella Di Cagno, Danilo Ercolini, Fabio Minervini, Marco Gobbetti, and Maria De Angelis, ‘Taxonomic Structure and Monitoring of the Dominant Lactic Acid Bacteria Population during Wheat Flour Sourdough Type I Propagation by using Lactobacillus sanfranciscensis Starters’, Appl. Environ. Microbiol., 2008, AEM.01524-08. Stolz, Peter, ‘Biological Fundamentals of Yeast and Lactobacilli Fermentation in Bread Dough’, in Handbook of Dough Fermentations (New York, NY: Marcel Dekker, Inc., 2003), pp. 23-42. Stolz, Peter, Georg Böcker, Rudi F. Vogel, and Walter P. Hammes, ‘Utilisation of maltose and glucose by lactobacilli isolated from sourdough’, FEMS Microbiology Letters, 109 (1993), 237-242. Thiele, C., S. Grassl, and M. Ganzle, ‘Gluten Hydrolysis and Depolymerization during Sourdough Fermentation’, Journal of Agricultural and Food Chemistry, 52 (2004), 13071314. 16