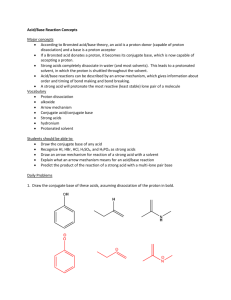
3. Acids and Bases Ka pKa log - = HCl + H2O H3O+ + Cl NH3 +
advertisement

3. Acids and Bases Most reactions can be categorized into acid-base or reduction-oxidation (redox) reactions. Chapter 3 will deal on acid-base reactions. The acids and bases described in the first involve proton (H+) transfer reaction (i.e., Brǿnsted acids and bases) Proton transfer equilibria can be discussed quantitatively in terms of acidity constants, which are a measure of the tendency to donate protons. In the second part, the definition is broadened to involve electron-pair sharing between a donor and an acceptor (i.e., Lewis acids and bases, will be explained next week). This broadening enables to extend acid-base reactions to species that do not contain protons and to nonaqueous media. 3.1. Brǿnsted acidity 3.1.1. Brǿnsted acids The original distinction between acids and bases was based on criteria of taste and feel: acids were sour and bases felt soapy. A typical example of an acid-base reaction is: HCl (acid) + NaOH (base) NaCl + H2O (neutralization) …(1) Johannes Brǿnsted and Thomas Lowry proposed (1923) that the essential feature of an acid–base reaction is the transfer of proton (H+). They suggested that a proton donor should be classified as a acid and a proton acceptor should be classified as a base. An example of a Brǿnsted acid is HCl, which can donate a proton to another molecule such as H2O: HCl + H2O H3O+ + Cl …(2) An example of a Brǿnsted base is ammonia, which can donate a proton to another molecule such as H2O: NH3 + H2O NH4+ + OH …(3) 3.1.2. Proton transfer equilibria in water Proton transfer between acids and bases is fast in both directions, so the dynamic equilibria, HCl + H2O NH3 + H2O H3O+ + Cl …(4) + NH4 + OH …(5) give a more complete description of the behavior of the acid HCl and the base NH3 in water than the forward reaction alone. 3.1.3. Conjugate acids and bases The form of the forward and reverse reactions given above (eq. 4), which depend on the transfer of a proton from an acid to a base is expressed by the general equilibrium: HA + H2O H3 O+ + …(6) + A is called the conjugate base of HA and H3O is called the conjugate acid of H2O. The conjugate base of an acid is the species that is left after a proton is lost. The conjugate acid of an base is the species that is formed when a proton is gained. There is no fundamental distinction between an acid and a conjugate acid or a base and a conjugate base. 3.1.4 The strengths of Brǿnsted acids The strength of a Brǿnsted acid, such as HCl, in aqueous solution is expressed by its acidity constant, Ka: HCl + H2O + H3O + Cl Ka [H 3 O ][Cl ] [HCl] pKa log Ka …(7) In eq. 8, [A] denotes the numerical value of the molar concentration of the species A. A value Ka >> 1 implies that [HA] is small respect to [A], and so proton transfer to water is preferred. (cf. Ka = 107 and pKa = 7 for HCl) Figure 25 summarizes the acidity constants of common acids and conjugate acids of some bases in water. A substance with pKa < 0 (corresponding to Ka > 1 and usually Ka >> 1) is a strong acid. Such acids are regarded as fully deprotonated in water. A substance with pKa > 0 (corresponding to Ka < 1) is a weak acid. For such species, proton transfer equilibrium lies in favor of nonionized acid. For example, HF is a weak acid in water, and hydrofluoric acid consists of hydronium ions, fluoride ions, and a high proportion of HF molecules. 3.1.5 Factors governing the strengths of acids and bases Figure 25. Acidity constants for species in aqueous solution at 298 K. A quantitative understanding of the relative acidities of H-A can be obtained by considering the enthalpy changes accompanying proton transfer. The simplest reaction of a proton is its attachment to a base A (could be a neutral molecule such as NH3) in the gas phase: A(g) + H+(g) HA(g) …(8) The standard enthalpy of this reaction is the proton-gain enthalpy pgH(A). The negative of this quantity is reported as proton affinity, Ap (Figure 26). When pgH is large and negative, corresponding to an exothermic proton attachment, the proton affinity is high, indicating the basic character of A. The proton affinities of the conjugate bases of HA decrease to the right along the period and down a group, indicating an increase in gas-phase acidity. Therefore, HF is a stronger acid than H2O, and HI is the strongest acid among the hydrogen halides. These trends can be explained by using a thermodynamic cycle (Figure 27), in which proton gain can be thought of as the outcome of three steps: Electron loss from A: A(g) A(g) + e(g) egH(A) = Ae(A) …(9) iH(H) = I(H) …(10) (the reverse of electron gain by A) Electron gain by H+ : H+(g) + e(g) H(g) (the reverse of ionization of H) Combination of H and A : H(g) + A(g) HA(g) B(H-A) …(11) (the reverse of H-A bond dissociation) The proton-gain enthalpy of A is the sum of these enthalpy changes: Overall+(g)(g) HA(g) pgH(A) = Ae(A) I(H) B(H-A) …(12) The dominant factor in the variation in proton affinity across a period is the trend in electron affinity of A, which increases from left to right, and hence lowers the proton affinity of A. Because the proton affinity of A decreases, the gas-phase acidity of HA increases across the period. The dominant factor when descending a group is the decrease of H-A bond dissociation enthalpy, which lowers the proton affinity of A and results in an increase in the gas-phase acidity of HA. The overall result of these effects is a decrease in gas-phase proton affinity of A-, and therefore an increase in the gas-phase acidity of HA, from the top left to bottom right of the p block (i.e., HI is a much stronger acid than CH4). Figure 26. Gas phase and solution proton affinities. Figure 27.Thermodynamic cycle for a proton gain reaction.