whmis homework booklet
advertisement

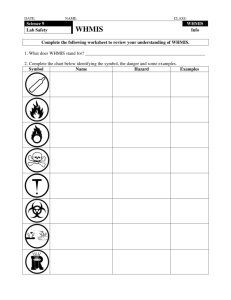
STUDENT NAME: ______________________ WHMIS HOMEWORK BOOKLET What is WHMIS and why is it important? WHMIS (Workplace Hazardous Material Information System) is a provincial legislative response to provincial employees' and employers' rights to know about the safety and health hazards of the materials they use in the workplace. This system and supporting documentation also provide sufficient information to ensure that people work safely with these materials. Teachers, students, custodians, and other staff may be exposed to hazardous materials. Such exposure can cause or contribute to serious health effects, such as effects on the nervous systems, kidney or lung damage, sterility, cancer, burns, and rashes. In addition, some hazardous materials may pose immediate physical or chemical risks, such as causing a fire or an explosion. WHMIS informs and protects school staff about such risks. Three major components to WHMIS include: ‣ Labels applied to containers of hazardous materials (called "controlled products" in the legislation). The labels alert the user to the dangers of the product and to the essential precautions for its safe use. ‣ Material Safety Data Sheets (MSDS) must be prepared by the product supplier and supplied to the user. These sheets provide detailed information about product composition, reactivity, health effects, protective equipment and procedures, and emergency procedures. Over sixty pieces of information, in nine separate sections, must be completed. Blanks are not permitted and a symbolic "N/A" must be clarified as "not applicable" (e.g., N/App) or "not available" (e.g., N/Av). Each MSDS must be dated and must not be more than three years old. See sample sheets for more detailed information. ‣ Education and Training related to hazards and associated safe work procedures is mandatory for those either working with or working in the proximity of the controlled product. The school is responsible to develop safe work procedures using knowledge of the job, the information from the labels, and the MSDS. It is expected that all teachers be trained sufficiently to use the information and training to protect themselves and their students. (Teachers must inform their principals of safe work procedures and insist that they are followed.) Note: The provisions of the WHMIS legislation with respect to laboratory chemicals (under 10 kg) are slightly different from those dealing with industrial chemicals. WHMIS legislation took effect throughout Canada on October 31, 1988. All controlled products sold after this date must meet the legislated labelling, MSDS, and worker education requirements or the product can not be used, handled, stored, or prepared for disposal. Combustible, corrosive, toxic, or highly reactive substances should be kept to a minimum and labelled as to their contents. The WHMIS definition of controlled product does not apply to radioactive materials, pesticides, explosives, consumer products, or materials covered under Food and Drug legislation (with respect to labels and MSDS, as a condition of sale). An equivalent amount of information is provided to workers to ensure the safe use of these products. Wood and tobacco (and products made of these substances), manufactured articles, and hazardous wastes are excluded from all aspects of WHMIS. Other provincial health and safety laws and regulations cover the hazards of these materials. Chemistry 20 - Period 2! Page! 1 STUDENT NAME: ______________________ Personal understanding: Create your own symbols There are a total of 6 WHMIS symbols; Compressed gas, Flammable and combustable material, Oxidizing material, Poisonous, Corrosive and Dangerously Reactive. In this activity I would like you to create a symbol for each of these categories. Do not worry about whether your symbol is the same as the MSDS symbol. I am asking you to design the symbol and I want to know what you would use as the symbol. Compressed Gas Flammable and combustable Material Oxidizing Material Poisonous Corrosive Dangerously Reactive Chemistry 20 - Period 2! Page! 2 STUDENT NAME: ______________________ WHMIS Classifications and Symbols The standard WHMIS symbols are below. For each symbol briefly explain your thoughts regarding the following: 1. Is the standard symbol clear? Can you understand the symbol with out an explanation? 2. What do you think about the standard WHMIS symbol compared to your symbol? ie: which symbol is easier to understand and why? 3. Having now seen the WHMIS symbol would you still have designed your symbol the same way and if so why? If you would change your symbol what would you change and why? Class A — Compressed Gas Class B — Flammable and Combustible Material Class C — Oxidizing Material Chemistry 20 - Period 2! Page! 3 STUDENT NAME: ______________________ Class D — Poisonous and Infectious Material 1. Materials Causing Immediate and Serious Toxic Effects Class D — Poisonous and Infectious Material 2.Materials causing other toxic effects Class D — Poisonous and Infectious Material 3.Biohazards infectious material Class E — Corrosive Material Class F — Dangerously Reactive Material Chemistry 20 - Period 2! Page! 4 STUDENT NAME: ______________________ Personal understanding: Thinking about Symbols Now that you understand the symbols think about some of the common chemicals you encounter each day in your home. Do you think these chemicals need a WHMIS symbol? What symbol, if any, do you think it has? Do you think the packaging has a symbol on it? Using the previous pages as a guide try to answer the following questions. When asked to indicate a specific symbol, or symbols use the class letter and number if necessary to designate a specific symbol. (For example: If you wanted to indicate “Dangerously Reactive Material: use the letter F, if you want to indicate “Biohazard” use the letter and number: D3” Chemical Does it need a symbol? What symbol do you think it is? Do you think it has a symbol on it? What symbol is on the container? White-out or liquid paper Rat Poison Bleach Propane Hair Spray Batteries Car Exhaust Shower Head Raw Chicken Coke Cola Toilet Bowl Cleaner Furniture Polish Spray paint Windshield washer fluid Now that you have considered some of the chemicals in your home go around and see if you can find these compounds. If you can find these compounds at your house check the symbol and fill in the last column of the table Chemistry 20 - Period 2! Page! 5 STUDENT NAME: ______________________ Labelling Proper labelling is one of the most important aspects of an effective and safe laboratory operation. Purchased stock chemicals kept in the storeroom and materials that are generated in the laboratory for and by student experiment require proper labelling. WHMIS requires increased information on labels of potentially hazardous materials. Labels warn handlers of potential hazards and therefore they must present the required information clearly and legibly. The formal "supplier label" contains seven elements of information inside a distinctively marked border ­ product identifier, supplier identifier, WHMIS hazard symbol(s), risk statement(s), precautionary statement(s), first aid information, and a reference to the MSDS. Laboratory chemicals from a recognized supply house may carry less information on the label (i.e., WHMIS symbols, distinctively marked border, and the supplier identifier). For example: Risk Phrases Product Identifier Hazard Symbol Precautionary Measure(s) First Aid Measures Reference to MSDS Supplier Identifier All chemical containers, including the original container, must be labelled in such a way as to identify the contents clearly. Inside the laboratory, small transfer containers and reaction vessels containing mixtures, solutions or reaction products must have a clear identifier, usually the chemical name. Outside the laboratory, transfer containers must carry a workplace label. This form of label has three components--the chemical identifier, instructions for safe use (combination of risk phrase and precautionary statement), and a reference to the MSDS. Chemistry 20 - Period 2! Page! 6 STUDENT NAME: ______________________ Additional Labelling Requirements Other legislation in Canada requires precautionary labelling on containers of hazardous materials (e.g., explosives, pesticides, or radioactive substances). Specific guidelines for the transportation of dangerous goods (TDG) have been developed to handle emergency response in the event of a spill or other accident under section D12 of the Dangerous Goods Handling and Transportation Act. These are also outlined in Prudent Practices for Handling Hazardous Chemicals in Laboratories published by National Academy Press in 1981. Transporting vehicles require diamond-shaped hazard placards. WHMIS is a complementary information system to the TDG regulations (consumer packaging is also regulated). In some jurisdictions, liability suits have been based on the inadequacy of a label on materials involved in an accident. Adequate labelling practices should help protect the teacher and school division/district from liability where labelling is the primary issue. TDG regulations do not cover the hazards of extended exposure in the workplace or the long-term effects of exposure. U.S. System of Labelling In 1977, the National Fire Protection Association (N.F.P.A.) in the United States developed a hazard coding system and an accompanying label. Although the system was not originally developed for use by laboratories, it has since become widely accepted in both industrial and educational laboratories throughout North America. It is a simple, easily understood coding system which identifies hazards associated with health, flammability, and reactivity of any given material as well as indicating special hazards associated with a substance. While WHMIS labels are the primary requirement in Canada, some chemicals come from the United States with the N.F.P.A. hazard diagram. They should be understood by Canadian users. The hazard label or diagram is divided into four coloured quadrants: ‣ The left hand quadrant (blue) indicates the relative health hazard. hazard. ‣ The top (red) quadrant indicates the flammability ‣ The (yellow) right hand quadrant indicates the Blue relative reactivity of the substance. ‣ The bottom quadrant (white) is used to indicate any special characteristics (e.g., oxidizing substances are identified with the letters OXY in the bottom segment, radiation hazards are indicated with the radiation symbol and water reactive substances have the letter W with a line drawn through the middle of it). ‣ The seriousness of any given hazard is indicated by the number located in the appropriate segment. Five digits from zero to four are used. A zero represents little or no hazard in that particular category, while substances with a four indicate extreme hazard (see illustration). Chemical containers sold in Canada in recent years White usually have N.F.P.A. labelling, but in some cases the colours in each quadrant are not present. Red Yellow It is important to note that the health hazard designations refer only to acute effects of exposure to the chemical. The long term, chronic effects are not taken into account. Chemistry 20 - Period 2! Page! 7 STUDENT NAME: ______________________ A complete listing is available from the N.F.P.A. Guide on Hazardous Materials (see Appendix J). Note: The N.F.P.A. system is provided as information only ­- it is not acceptable by itself (WHMIS is the Canadian standard). Category One - Health (Blue) ‣ ‣ ‣ ‣ ‣ 4 - Can cause death or major injury despite medical treatment. 3 - Can cause serious injury despite medical treatment. 2 - Can cause injury. Requires prompt treatment. 1 - Can cause irritation if not treated. 0 - No hazard. Category Two ­ Fire (Red) ‣ ‣ ‣ ‣ ‣ 4 - Very flammable gases or very volatile flammable liquids. 3 - Can be ignited at all normal temperatures. 2 - Ignites if moderately heated. 1 - Ignites after considerable preheating. 0 - Will not burn. Special Category Three ­ Reactivity (Stability) (Yellow) ‣ ‣ ‣ ‣ ‣ 4 - Readily detonates or explodes. 3 - Can detonate or explode but requires strong initiating force or heating under confinement. 2 - Normally unstable but will not detonate. 1 - Normally stable. Unstable at high temperature and pressure. Reacts with water. 0 - Normally stable. Not reactive with water. Key to Hazard (Special) Comments ‣ ‣ ‣ ‣ ‣ ‣ ‣ ‣ C - may be carcinogenic upon chronic exposure Cor ­ corrosive Exp - risk of explosion Oxy ­ oxidizing agent SC ­ suspected carcinogen T ­ toxic W­ water reactive Pol ­ polymerizes under normal conditions Chemistry 20 - Period 2! Page! 8 STUDENT NAME: ______________________ Personal understanding: MSDS Workplace labels Choose a regular item you encounter each day and create an MSDS workplace label for this item. This could be your best friend, pet dog, favorite game console or favorite pool cue. Once you have selected an item create a work place MSDS label for this item. Remember to include the product identifier along with supplier information, hazard symbols, precautionary statements and first aid information. If there is insufficient room with in the space provided feel free to use a separate sheet of paper. REFER TO MATERIAL SAFETY DATA SHEET FOR FURTHER INFORMATION Chemistry 20 - Period 2! Page! 9 STUDENT NAME: ______________________ Material Safety Data Sheets Material Safety Data Sheets (MSDS) must be supplied with hazardous chemicals and may be requested for other chemicals. Teachers and students should be familiar with the type of information contained on a MSDS and be able to interpret the sheets from a variety of chemical suppliers. Although the numbering of sections and the order of appearance may differ from supplier to supplier, the following must be on each MSDS: 1. Product Identification and Use Manufacturer's name Supplier's name 2. Hazardous Ingredients 3. Physical Data Colour, form, solubility Melting and boiling points Vapour pressure, specific gravity 4. Fire and Explosion Data Flammability Flashpoint Fire fighting procedures 5. Reactivity Data Stability and Hazards 6. Toxicological Properties Threshold Limit Values (TLV) Effects of exposure Carcinogenicity 7. Preventative Measures Protective clothing Protective equipment Spill and handling procedures 8. First Aid Measures 9. Preparation Date of MSDS 10.Teaching About MSDS Chemistry 20 - Period 2! Page!10 STUDENT NAME: ______________________ Personal understanding: Reading MSDS sheets Using the information sheet for caffeine which is included on the following pages answer the questions below. For each answer please reference the section you found it in. QUESTIONS: 1. What is the chemical name of Caffeine? 2. What is the Chemical Formula for Caffeine? 3. What are the side affect of Caffeine ingestion? 4. What do you think caffeine tastes like? Why? 5. What is the personal protective equipment you should be wearing while working with Caffeine? 6. What are the effects which can be expected with chronic exposure? 7. What is the recommended first aid procedure should someone ingest Caffeine? 8. Does caffeine pose a fire or explosive risk? 9. At what temperature will caffeine burst into flame? What temperature does water boil at? 10.Why doesn’t caffein in tea burn? 11.What color is caffeine? 12.LD50 means the does which will kill 50% of a given population. What is the LD50 for a rat? 13.what would be the LD50 dose for a 5kg rat? 14.How much caffeine is in coke? How about red bull? (You may need to use the internet for this one) 15.What is your LD50 dose (1Kg = 2.2lbs)? Chemistry 20 - Period 2! Page!11 STUDENT NAME: ______________________ MSDS Number: C0165 * * * * * Effective Date: 09/16/09 * * * * * Supercedes: 08/03/07 CAFFEINE Section 1: Chemical Product and Company Identification Synonyms: 1,3,7-trimethylxanthine CAS No.: 58-08-2 Molecular Weight: 194.19 Chemical Formula: C8H10N4O2 Product Codes: E268 Section 2: Composition and Information on Ingredients Composition: ! Name! CAS#! % by Weight ! Caffeine! 58-08-2! 100 Toxicological Data on Ingredients: Caffeine: ORAL (LD50): Acute: 192 mg/kg [Rat]. 127 mg/kg [Mouse]. 224 mg/kg [ Rabbit]. Chemistry 20 - Period 2! Page!12 STUDENT NAME: ______________________ Section 3: Hazards Identification Potential Acute Health Effects: Hazardous in case of skin contact (irritant), of eye contact (irritant), of ingestion, of inhalation. Severe over-exposure can result in death. Potential Chronic Health Effects: CARCINOGENIC EFFECTS: 3 (Not classifiable for human.) by IARC. MUTAGENIC EFFECTS: Mutagenic for mammalian somatic cells. Mutagenic for bacteria and/or yeast. TERATOGENIC EFFECTS: Not available. DEVELOPMENTAL TOXICITY: Not available. The substance may be toxic to heart, gastrointestinal tract, central nervous system (CNS). Repeated or prolonged exposure to the substance can produce target organs damage. Repeated exposure to a highly toxic material may produce general deterioration of health by an accumulation in one or many human organs. Section 4: First Aid Measures Eye Contact: Check for and remove any contact lenses. In case of contact, immediately flush eyes with plenty of water for at least 15 minutes. Cold water may be used. WARM water MUST be used. Get medical attention. Skin Contact: In case of contact, immediately flush skin with plenty of water. Cover the irritated skin with an emollient. Remove contaminated clothing and shoes. Wash clothing before reuse. Thoroughly clean shoes before reuse. Get medical attention. Serious Skin Contact: Wash with a disinfectant soap and cover the contaminated skin with an anti-bacterial cream. Seek immediate medical attention. Inhalation: If inhaled, remove to fresh air. If not breathing, give artificial respiration. If breathing is difficult, give oxygen. Get medical attention. Serious Inhalation: Evacuate the victim to a safe area as soon as possible. Loosen tight clothing such as a collar, tie, belt or waistband. Seek medical attention. Ingestion: If swallowed, do not induce vomiting unless directed to do so by medical personnel. Never give anything by mouth to an unconscious person. Loosen tight clothing such as a collar, tie, belt or waistband. Get medical attention immediately. Serious Ingestion: Not available. Section 5: Fire and Explosive Data Flammability of the Product: May be combustible at high temperature. Auto-Ignition Temperature: Not available. Flash Points: CLOSED CUP: Higher than 93.3°C (200°F). Flammable Limits: Not available. Products of Combustion: These products are carbon oxides (CO, CO2), nitrogen oxides (NO, NO2...). Fire Hazards in Presence of Various Substances: Slightly flammable to flammable in presence of open flames and sparks, of heat. Non-flammable in presence of shocks. Explosion Hazards in Presence of Various Substances: Risks of explosion of the product in presence of mechanical impact: Not available. Risks of explosion of the product in presence of static discharge: Not available. Fire Fighting Media and Instructions: SMALL FIRE: Use DRY chemical powder. LARGE FIRE: Use water spray, fog or foam. Do not use water jet. Special Remarks on Fire Hazards: Not available. Special Remarks on Explosion Hazards: Not available. Chemistry 20 - Period 2! Page!13 STUDENT NAME: ______________________ Section 6: Accidental Release Measures Small Spill: Use appropriate tools to put the spilled solid in a convenient waste disposal container. Large Spill: Poisonous solid. Stop leak if without risk. Do not get water inside container. Do not touch spilled material. Use water spray to reduce vapors. Prevent entry into sewers, basements or confined areas; dike if needed. Eliminate all ignition sources. Call for assistance on disposal. Section 7: Handling and Storage Precautions: Keep locked up.. Keep away from heat. Keep away from sources of ignition. Empty containers pose a fire risk, evaporate the residue under a fume hood. Ground all equipment containing material. Do not ingest. Do not breathe dust. Wear suitable protective clothing. In case of insufficient ventilation, wear suitable respiratory equipment. If ingested, seek medical advice immediately and show the container or the label. Avoid contact with skin and eyes. Storage: Keep container tightly closed. Keep container in a cool, well-ventilated area. Section 8: Exposure Controls / Personal Protection Engineering Controls: Use process enclosures, local exhaust ventilation, or other engineering controls to keep airborne levels below recommended exposure limits. If user operations generate dust, fume or mist, use ventilation to keep exposure to airborne contaminants below the exposure limit. Personal Protection: Splash goggles. Lab coat. Dust respirator. Be sure to use an approved/certified respirator or equivalent. Gloves. Personal Protection in Case of a Large Spill: Splash goggles. Full suit. Dust respirator. Boots. Gloves. A self contained breathing apparatus should be used to avoid inhalation of the product. Suggested protective clothing might not be sufficient; consult a specialist BEFORE handling this product. Exposure Limits: Not available. Section 9: Physical and Chemical Properties Physical state and appearance: Solid. (Crystalline solid.) Odor: Odorless. Taste: Bitter. (Slight.) Molecular Weight: 194.2 g/mole Color: White. pH (1% soln/water): 6.9 [Neutral.] Boiling Point: Not available. Melting Point: 238°C (460.4°F) Critical Temperature: Not available. Specific Gravity: 1.23 (Water = 1) Vapor Pressure: Not applicable. Vapor Density: Not available. Volatility: Not available. Odor Threshold: Not available. Water/Oil Dist. Coeff.: The product is equally soluble in oil and water; log(oil/water) = -0.1 Ionicity (in Water): Not available. Dispersion Properties: See solubility in water, diethyl ether. Solubility: Soluble in hot water. Partially soluble in cold water, acetone. Very slightly soluble in diethyl ether. Freely soluble in pyrrole; in tetrahydrofuran containing about 4% water. Soluble in pyridine, ethyl acetate. Partially soluble in alcohol Slightly soluble in petroleum ether. Solubility in water is increased by alkali benzoates, cinnamates, citrates or salicylates p. 3Section 10: Stability and Reactivity Data Stability: The product is stable. Instability Temperature: Not available. Conditions of Instability: Excess heat Incompatibility with various substances: Not available. Corrosivity: Non-corrosive in presence of glass. Special Remarks on Reactivity: Not available. Special Remarks on Corrosivity: Not available. Polymerization: Will not occur. Chemistry 20 - Period 2! Page!14 STUDENT NAME: ______________________ Section 10: Stability and Reactivity Data Stability: The product is stable. Instability Temperature: Not available. Conditions of Instability: Excess heat Incompatibility with various substances: Not available. Corrosivity: Non-corrosive in presence of glass. Special Remarks on Reactivity: Not available. Special Remarks on Corrosivity: Not available. Polymerization: Will not occur. Section 11: Toxicological Information Routes of Entry: Inhalation. Ingestion. Toxicity to Animals: Acute oral toxicity (LD50): 127 mg/kg [Mouse]. Chronic Effects on Humans: CARCINOGENIC EFFECTS: 3 (Not classifiable for human.) by IARC. MUTAGENIC EFFECTS: Mutagenic for mammalian somatic cells. Mutagenic for bacteria and/or yeast. May cause damage to the following organs: heart, gastrointestinal tract, central nervous system (CNS). Other Toxic Effects on Humans: Hazardous in case of skin contact (irritant), of ingestion, of inhalation. Special Remarks on Toxicity to Animals: LDL [Human] - Route: Oral; Dose: 192mg/kg LDL [Woman] - Route: Oral; Dose; 400 mg/kg LDL [Child] - Route: Oral; Dose: 320 mg/kg Special Remarks on Chronic Effects on Humans: May cause adverse reproductive effects (fetotoxicity, maternal (parnutrition) and birth defects. May affect genetic material (mutagenic). May cause cancer (tumorgenic) based on animal data. Special Remarks on other Toxic Effects on Humans: Acute Potential Health Effects: Skin: May cause skin irritation. Eyes: Dust may cause mechanical irritation. Inhalation: May cause respiratory tract irritation. Ingestion: Harmful if swallowed in large amounts. May cause gastrointestinal (digestive) tract irritation with epigastric pain, abdominal cramps, nausea, vomiting and diarrhea. Affects metabolism and cardiovascular system with symptoms including increase in metabolism, flushing, palpitations, rapid heart rate, dysrhythmias, hypotension, blood pressure elevation and weight loss, metabolic acidosis. May affect brain and behavior/central nervous system.. Symptoms may include nervousness, anxiety, restlessness, insomnia, dizziness, tremor, seizures, convulsions, hallucinations, somnolence, toxic psychosis, tremors, convulsions, ataxia. May also affect blood, respiration (hyperventilation), and urinary system (mild increase in urinary volume and urinary sodium excretion), and may directly produce hypokalemia. Chronic Potential Health Effects: May cause cancer (tumorigen) based on animal studies. May cause reproductive and fetal effects. May cause digestive tract disturbances (increased gastric acid, and pepsin secretion and a decrease in lower esophogeal sphincter pressure), cardiovascular disturbances. Since it is a CNS stimulant, it may also affect the Central Nervous System (CNS). Section 12: Ecological Information Ecotoxicity: Not available. BOD5 and COD: Not available. Products of Biodegradation: Possibly hazardous short term degradation products are not likely. However, long term degradation products may arise. p. 4Toxicity of the Products of Biodegradation: The products of degradation are less toxic than the product itself. Special Remarks on the Products of Biodegradation: Not available. Section 13: Disposal Considerations Waste Disposal: Waste must be disposed of in accordance with federal, state and local environmental control regulations Section 14: Transport Information DOT Classification: CLASS 6.1: Poisonous material. Identification: : Alkaloid, solid, n.o.s. (Caffeine) UNNA: 1544 PG: III Special Provisions for Transport: Not available Chemistry 20 - Period 2! Page!15 STUDENT NAME: ______________________ Section 15: Other Regulatory Information Federal and State Regulations: TSCA 8(b) inventory: Caffeine Other Regulations: OSHA: Hazardous by definition of Hazard Communication Standard (29 CFR 1910.1200). EINECS: This product is on the European Inventory of Existing Commercial Chemical Substances. Other Classifications: WHMIS (Canada): Not controlled under WHMIS (Canada). Does not have a WHMIS classifcation. DSCL (EEC): R22- Harmful if swallowed. R40- Possible risks of irreversible effects. R63- Possible risk of harm to the unborn child. S2- Keep out of the reach of children. HMIS (U.S.A.): Health Hazard: 2 Fire Hazard: 1 Reactivity: 0 Personal Protection: E National Fire Protection Association (U.S.A.): Health: 2 Flammability: 1 Reactivity: 0 Specific hazard: Protective Equipment: Gloves. Lab coat. Dust respirator. Be sure to use an approved/certified respirator or equivalent. Splash goggles Section 16: Other Information References: Not available. Other Special Considerations: Not available. Section 13: Disposal Considerations Waste Disposal: Waste must be disposed of in accordance with federal, state and local environmental control regulations. Section 14: Transport Information DOT Classification: CLASS 6.1: Poisonous material. Identification: : Alkaloid, solid, n.o.s. (Caffeine) UNNA: 1544 PG: III Special Provisions for Transport: Not available. Section 15: Other Regulatory Information Federal and State Regulations: TSCA 8(b) inventory: Caffeine Other Regulations: OSHA: Hazardous by definition of Hazard Communication Standard (29 CFR 1910.1200). EINECS: This product is on the European Inventory of Existing Commercial Chemical Substances. Other Classifications: WHMIS (Canada): Not controlled under WHMIS (Canada). Does not have a WHMIS classifcation. DSCL (EEC): R22- Harmful if swallowed. R40- Possible risks of irreversible effects. R63- Possible risk of harm to the unborn child. S2- Keep out of the reach of children. HMIS (U.S.A.): Health Hazard: 2 Fire Hazard: 1 Reactivity: 0 Personal Protection: E National Fire Protection Association (U.S.A.): Health: 2 Flammability: 1 Reactivity: 0 Specific hazard: Protective Equipment: Gloves. Lab coat. Dust respirator. Be sure to use an approved/certified respirator or equivalent. Splash goggles. Section 16: Other Information p. 5Created: 10/11/2005 11:30 AM Last Updated: 11/06/2008 12:00 PM The information above is believed to be accurate and represents the best information currently available to us. However, we make no warranty of merchantability or any other warranty, express or implied, with respect to such information, and we assume no liability resulting from its use. Users should make their own investigations to determine the suitability of the information for their particular purposes. In no event shall ScienceLab.com be liable for any claims, losses, or damages of any third party or for lost profits or any special, indirect, incidental, consequential or exemplary damages, howsoever arising, even if ScienceLab.com has been advised of the possibility of such damages Chemistry 20 - Period 2! Page!16