Chapter 19: Citric Acid Cycle
advertisement

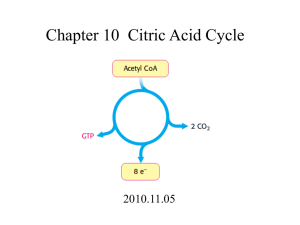
Takusagawa’s Note 1 Chapter-16 Chapter 16: Citric Acid Cycle 1. CITRIC ACID CYCLE OVERVIEW (all carried out in mitochondrial matrix) - Citric acid cycle is also called Krebs cycle or tricarboxylic acid (TCA) cycle. - Citric acid cycle is the process that the pyruvate produced in glycolysis is further oxidized to 3CO2 to produce 4NADH, FADH2 and GTP (ATP). The NADH and FADH2 are utilized to produce the “energy currency” ATP in oxidative phosphorylation. O Pyruvate H3C C COO CoASH + NAD+ pyruvate dehydrogenase CO2 + NADH O H3C C S CoA Acetyl-CoA NADH + H+ COO+ NAD C O - COO HO CH L-malate CH2 COO7 H2O H2O - COO CoASH COO- cis-Aconitate CH COO- CO2 COOIsocitrate C O 3 C O COO- 3 CH2 4 CH2 NAD + C O COO- CO2 α-Ketoglutarate 2CO2 are not come from the original acetate. 1 CH2 H C COO HO C H COO- CH2 H C COO CoASH COO- H2O 2 COO CH2 S CoA Succinyl-CoA NADH + H+ • COOCH2 C COO - CH2 GDP + Pi H2O 2 7. fumarase 8. malate dehydrogenase Succinate CH2 CH2 5 Citrate 4. α-ketoglutarate dehydrogenase 5. succinyl-CoA synthetase 6. Succinate dehydrogenase 6 GTP CH2 HO C COO CH2 COO- 3. isocitrate dehydrogenase COO- COO- 1 CH2 COOOxaloacetate 1. citrate synthase 2. aconitase Fumarate CH HC FAD CoA 8 COO- FADH2 COO- Oxalosuccinate NAD+ NADH + H+ Takusagawa’s Note 2 Chapter-16 - Pyruvate generated from glycolysis is converted to acetyl-CoA before entering the citric acid cycle. - At the initial reaction, acetyl group from acetyl-CoA and oxaloacetate react to form citrate. - 3NADH, FADH2 and GTP are generated from one acetyl-CoA oxidation. - 2CO2 are released from the portion of oxaloacetate. - At the final reaction, oxaloacetate is regenerated. - Overall reaction in the citric acid cycle is: 3NAD+ + FAD + GDP + Pi + acetyl-CoA → 3NADH + FADH2 + GTP + CoA + 2CO2 From glucose: Glucose + 2NAD+ + 2ADP + 2Pi → 2pyruvate + 2NADH + 2ATP 2pyruvate + 2NAD+ + 2CoA → 2acetyl-CoA + 2NADH + 2CO2 2acetyl-CoA + 6NAD+ + 2FAD + 2GDP + 2Pi → 6NADH + 2FADH2 + 2GTP + 2CoA + 4CO2 2GTP + 2ADP → 2ATP + 2GDP . Glucose + 10NAD+ + 4ADP + 4Pi + 2FAD → 10NADH + 2FADH2 + 4ATP + 6CO2 → 30ATP + 4ATP + 4ATP = 38ATP 2. METABOLIC SOURCES OF ACETYL-COENZYME A - Pyruvate is converted to acetyl-CoA before entering the citric acid cycle. - The function of coenzyme A is a carrier of acetyl and other acyl group. - Acetyl-CoA is a “high-energy” compound since it has a “high energy” S~C bond which releases ∆G°’ = -31.5 kJ/mol by hydrolysis. Acetyl group O S C CH 3 β-mercaptoethylamine residue High energy bond CH2 CH2 NH C O Adenosine-3'phosphate CH2 CH2 NH2 NH Pantothenic acid residue C O N N HO C H H3C C CH3 H2C O O N N O P O P O CH2 O- O- O O OH - - O P O 2 Acetyl-coenzyme A (acetyl-CoA) O Chapter-16 3 Takusagawa’s Note A. Pyruvate dehydrogenase is a multienzyme complex - Acetyl-CoA is formed from pyruvate through oxidative decarboxylation by a multienzyme complex named pyruvate dehydrogenase. Pyruvate + CoA + NAD+ → acetyl-CoA + CO2 + NADH - Pyruvate dehydrogenase multienzyme complex consists of: 1. Pyruvate dehydrogenase (E1) 2. Dihydrolipoyl transacetylase (E2) 3. Dihydrolipoyl dehydrogenase (E3) - Multienzyme complexes have catalytic advantages: 1. Rates of a series of reactions are enhanced since short diffusion distance. 2. Side reactions are minimized. 3. Reactions may be coordinately controlled. - The following coenzymes and prosthetic groups are required in pyruvate dehydrogenase multienzyme complex: - Thiamine pyrophosphate (TPP, Fig. 16-27) decarboxylase - Flavin adenine dinucleotide (FAD, Fig. 14-28) redox See next page. + - Nicotinamide adenine dinucleotide (NAD , Fig. 12-2) redox - Coenzyme A (see previous page) acetyl-carrier - Lipoamide (prosthetic group) acetyl-carrier 3 Chapter-16 4 4 Takusagawa’s Note Chapter-16 5 Takusagawa’s Note Acetyl-CoA formation occurs in five reactions 1. Pyruvate dehydrogenase (E1), a TPP-requiring enzyme, decarboxylates a pyruvate with the intermediate formation of hydroxyethyl-TPP. This is the same reaction catalyzed by yeast pyruvate decarboxylase (pyruvate → acetylaldehyde + CO2). 5 Takusagawa’s Note 6 Chapter-16 2. Hydroxyethyl group is transferred to the next enzyme (dihydrolipoyl transacetylase (E2)). The hydroxyethyl group carbanion attacks the lipoamide disulfide of E2 and eliminate the TPP to form acetyl-dihydrolipoamide-E2. + H B: 3. The acetyl group is transferred to CoA to yield acetyl-CoA and dihydrolipoamide-E2. 4. Dihydrolipoamide-E2 is oxidized by dihydrolipoyl dehydrogenase (E3) + + 5. Reduced E3 is reoxidized by NAD+. Initially the enzyme’s sulfhydryl groups (-SH) are reoxidized by the enzyme-bound FAD, yielding FADH2, then FADH2 is reoxidized by NAD+, producing NADH. 6 Takusagawa’s Note 7 Chapter-16 The lipoyllysyl arm transfers intermediates between enzyme subunits - Lipoyllysyl arm is quite long (14 Å). 14 Å O O HN N H S S Lipollysyl arm (fully extended) Arsenic compounds are poisonous because they covalently bind to the vicinal (adjacent) dithiols of dihydrolipoamide. 7 Chapter-16 8 Takusagawa’s Note B. Control of pyruvate dehydrogenase Product inhibition - When the relative concentrations of NADH and acetyl-CoA are high, the reversible reactions catalyzed by E2 and E3 are driven backwards. Therefore formation of acetyl-CoA is inhibited. - Thus the E2 and E3 activities are controlled by product inhibition (acetyl-CoA for E2 and NADH for E3). Covalent modification (Eukaryotic complex only) - E1 is regulated by phosphorylation/dephosphorylation. When the Ser of E1 is phosphorylated, the enzyme is inactivated. Insulin activates Activators of phosphatase: Mg2+, Ca2+ Activators of kinase: Acetyl-CoA, NADH Inhibitors of kinase: Pyruvate, ADP, Ca2+, high Mg2+, K+ - Remember: Insulin inhibits phosphorylation and activates dephosphorylation in order to reduce the [glucose] in blood at the starting point of glycolysis. Now, insulin also works to reduce the end product of glycolysis, i.e., activates dephosphorylation of E1 to convert pyruvate to acetyl-CoA. Acetyl-CoA is not only the fuel of citric acid cycle, but also the precursor of fatty acids. 8 Takusagawa’s Note 9 Chapter-16 3. Enzymes of the citric acid cycle A. Citrate synthase - catalyzes the condensation of acetyl-CoA and oxaloacetate. O O + H3C C S CoA Acetyl-CoA H2O - O C COO H2C CoA-SH - H2C C O - HO C COO - H2C COO - COO Citrate Oxaloacetate ∆G°’ = -32.2 kJ/mol Reaction mechanism 1. Asp-375 acts as a base to remove a proton from the methyl group of acetyl-CoA. His-274 acts as an acid to protonate the enolate oxygen. 2. Citryl-CoA is formed in a second concerted acid-base catalysis. His-320 acts as acid, and His-274 acts as base. 3. Citryl-CoA is hydrolyzed to citrate and CoA. This hydrolysis (∆G°’ = -31.5 kJ/mol) pulls the reaction 1 and 2. 1 CoASH 3 9 H2O Takusagawa’s Note 10 Chapter-16 B. Aconitase - catalyzes the reversible isomerization of citrate and isocitrate. - H2C COO Citrate H 2O - H2C COO - H C COO C COO - C COO H - H2C COO - HO C COO H C H 2O - COO - HO C - COO H H cis-Aconitate Isocitrate ∆G°’ = 13.3 kJ/mol Reaction mechanism - Aconitase contains a covalently bound [4Fe-4S] iron-sulfur cluster, which is required for catalytic activity. The Fea is coordinated by the hydroxyl and the central carboxyl groups. 1. His-101 acts as an acid to eliminate -OH as water, and Ser-642 acts as a base to eliminate a proton from C2. 2. cis-Aconitate intermediate is flipped by 180° so that C2 and C3 are exchanged their positions. 3. The reversed acid-base catalysis is taken place to yield (2R,3S)-isocitrate. 10 Takusagawa’s Note 11 Chapter-16 Fluorocitrate inhibits aconitase - Fluoroacetate, one of the most toxic small molecules (LD50 = 0.2 mg/kg), is converted to (2R,3R)-fluorocitrate, which specifically inhibits aconitase since Ser-642 cannot remove the proton at C2. Less acidic Less toxic Very toxic C. NAD+-dependent isocitrate dehydrogenase - catalyzes the oxidative decarboxylation of isocitrate to α-ketoglutarate to produce CO2 and NADH. - H2C COO NAD + NADH + H + - H2C COO - HO C C COO CO2 - COO O H α-Ketoglutarate Isocitrate - + CH2 H C COO ∆G°’ = -20.9 kJ/mol There are two isozymes in mammalian cells. 1. NAD+-dependent form is in mitochondria and requires an Mn2+ or Mg2+. 2. NADP+-dependent form is in both cytosol and mitochondria. D. α-Ketoglutarate dehydrogenase - catalyzes the oxidative decarboxylation of an α-keto acid, releasing CO2, forming succinylCoA and reducing NAD+ to NADH. + H2C COO CoA-SH NAD NADH CH2 C - H2C COO CH2 - COO C O + S-CoA O α-Ketoglutarate Succinyl-CoA ∆G°’ = -33.5 kJ/mol 11 CO2 - - Takusagawa’s Note 12 Chapter-16 α-Ketoglutarate dehydrogenase is a multienzyme complex that consists of α-ketoglutarate dehydrogenase (E1), dihydrolipoyl transsuccinylase (E2), and dihydrolipoyl dehydrogenase (E3). The overall reaction closely resembles that are catalyzed by the pyruvate dehydrogenase multienzyme complex, i.e., 1. Decarboxylation -----------------------E1 E2 2. Succinyl group transfer 3. Succinyl-CoA formation. E3 4. Oxidation of E2. + 5. Reduction of NAD . E. Succinyl-CoA synthetase - hydrolyzes the “high-energy” compound succinyl-CoA with the coupled synthesis of a “highenergy” nucleosidetriphosphate (GTP). H2C COO- CH2 GDP + Pi GTP - COO CoA-SH CH2 C S-CoA CH2 - O COO Succinate Succinyl-CoA ∆G°’ = -2.9 kJ/mol - The succinyl~CoA thioester bond energy is preserved through the formation of a series of “high-energy” phosphate (~Pi). The succinate formation is as follows: Pi Succinyl~CoA 1 GDP~Pi (GTP) CoASH Succinyl~Pi 2 3 E-His~Pi E-His GDP - Succinate GTP is converted to ATP by nucleoside diphosphate kinase. ∆G°’ = 0 kJ/mol GTP + ADP ↔ GDP + ATP 12 E-His Takusagawa’s Note 13 Chapter-16 F. Succinate dehydrogenase - catalyzes stereospecific dehydrogenation of succinate to fumarate and produces FADH2. - - FADH2 FAD COO COO C H H C H H C H C H - - COO Fumarate COO Succinate - - ∆G°’ = 0 kJ/mol The FAD in succinate dehydrogenase is covalently bound to the enzyme. Thus, FADH2 cannot be oxidized as a cofactor. FADH2 is oxidized by the electron transport chain reaction (See Chapter-17). For the reason, succinate dehydrogenase is the only membrane-bound citric acid cycle enzyme. The others are dissolved in the mitochondrial matrix. The enzyme is strongly inhibited by malonate (structural analog of succinate). - COO - COO H C H H C H H C H - COO - COO Malonate Succinate + In general, FAD and NAD are involved in different oxidation-reduction reactions. - For example, FAD FADH2 H C H C H H C H H C Alkane Alkene - The oxidation of alkane to alkene produces ∆G°’ ≈ -42 kJ/mol, whereas the FAD to FADH2 reduction requires ~42 kJ/mol (FAD + 2H+ + 2e- → FADH2, ∆E°’ = -0.219 V = (∆G°’ = 42 kJ/mol)). Thus, the oxidation of alkane to alkene is just enough to reduce FAD to FADH2, but not enough to reduce NAD+ to NADH + H+ (∆G°’ = 61 kJ/mol). - The oxidation of alcohol to aldehyde (or ketone) produces more energy than the above case. NAD H C H H C OH Alcohol - + + NADH + H H C H C O Aldehyde or ketone Alcohol → aldehyde (or ketone) ∆G°’ ≈ -61 kJ/mol + + + NAD + 2H + 2e → NADH + H ∆E°’ = -0.315 V (∆G°’ = 61 kJ/mol) The oxidation of alcohol to aldehyde is sufficient to reduce NAD+ to NADH2. 13 Takusagawa’s Note 14 Chapter-16 G. Fumarase - catalyzes the hydration of fumarate’s double bond to form L-malate. - COO H2O - COO C H HO C H H C H C H - - COO COO L-Malate Fumarate ∆G°’ = -3.8 kJ/mol H. Malate dehydrogenase - catalyzes the oxidation of L-malate’s hydroxyl group to ketone in a NAD+-dependent reaction, regenerating oxaloacetate. - COO NAD + NADH + H - COO HO C H C O H C H H C H - COO - COO L-Malate - + Oxaloacetate ∆G°’ = 29.7 kJ/mol This reaction is relatively high endergonic reaction (∆G > 0). However, the following two reasons, this reaction occurs. 1. [Oxaloacetate] is very low at equilibrium, i.e., RTlnKeq becomes negative where [oxaloacetate][ NADH] < 1, i.e., lnK < 0. Keq = eq [ malate] NAD + [ ] 2. The subsequent reaction (formation of citrate from oxaloacetate and acetyl-CoA) that is highly exergonic pulls this reaction since the hydrolysis of “high-energy” thioester bond of acetyl-CoA releases ∆G°’ = -31.5 kJ/mol energy. This is a reason why acetyl-CoA enters the citric acid cycle. 14 Chapter-16 15 Takusagawa’s Note I. Integration of the citric acid cycle - Citric acid cycle results in the following chemical transformations. 1. One acetyl group (-COCH3) → 2CO2 (4-electron pair process). O - + CoA S C CH3 + 3H2O 2CO2 + CoA SH + 8H + 8e 2. Reduction of three NAD+ to three NADH (3-electron pairs process) and equivalent to 9ATP generation, i.e., 3NAD+ + 6H+ + 6e- → 3NADH + 3H+ 3. Reduction of one FAD to FADH2 (1-electron pairs process) and equivalent to 2ATP generation, i.e., FAD + 2H+ + 2e- → FADH2 4. Generation of one GTP (ATP). Four electron pairs generated by one acetyl group oxidation are carried by 3NADH and FADH2 to the oxidative phosphorylation pathway to generate 11ATP. Thus, citric acid cycle generates 12ATP from one acetyl group and sends 4-electron pairs (8 electrons) to electron-transport chain, where they reduce two molecules of O2 to 4H2O, i.e., 2O2 + 8H+ + 8e- → 4H2O. 15 Chapter-16 16 Takusagawa’s Note 4. REGULATION OF THE CITRIC ACID CYCLE - Citrate synthase, isocitrate dehydrogenase and α-ketoglutarate dehydrogenase are the citric acid cycle’s rate-controlling enzymes because those ∆G are negative. - The citric acid cycle reactions are carried out in mitochondria, but most of the cycle’s metabolites are present in both mitochondria and cytosol. Therefore it is difficult to establish the rate-determining steps. - However, three of the eight steps have significantly negative physiological free energy changes. The enzymes involved in those steps are likely to function far from equilibrium under physiological conditions. Standard (∆G°’) and physiological (∆G) free energy changes Reaction Enzyme ∆G°’ (kJ/mol) 1 Citrate synthase -32.2 2 Aconitase +13.3 3 Isocitrate dehydrogenase -20.9 -33.5 4 α-Ketoglutarate dehydrogenase 5 Succinyl-CoA synthetase -2.9 6 Succinate dehydrogenase 0.0 7 Fumarase -3.8 8 Malate dehydrogenase +29.7 - ∆G (kJ/mol) Negative ~0 Negative Negative ~0 ~0 ~0 ~0 Unlike enzymes in glycolysis and glycogen metabolism, the citric acid cycle is largely regulated by 1. substrate availability (rate of diffusion of substrate into mitochondria) 2. product inhibition. (NADH, ATP, citrate) 3. competitive feedback inhibition by intermediates further along the cycle. 16 Chapter-16 17 Takusagawa’s Note Products and NADH are involved in feedback inhibition. - ADP and ATP are allosteric regulators of isocitrate dehydrogenase. High [ADP] activates the enzyme whereas high [ATP] inhibits the enzyme. - Ca2+ activates pyruvate dehydrogenase, isocitrate dehydrogenase and α-ketoglutarate dehydrogenase. 17 Takusagawa’s Note 18 Chapter-16 5. THE AMPHIBOLIC NATURE OF THE CITRIC ACID CYCLE - In the muscle, the citric acid cycle works mainly degradation of acetyl-CoA to produce bioenergies (ATP). - In the liver, the citric acid cycle is amphibolic. Note: Amphibolic = both anabolic and catabolic processes. Anabolism: Amino acids Sugars Fatty acids, etc. Catabolism: Energy yielding materials, such as proteins ⇒ Proteins Nucleic acids Lipids, etc. ⇒ Energy poor end products, such as CO2, NH3, H2O Intermediates of citric acid cycle are also various precursors 18 Chapter-16 - 19 Takusagawa’s Note Intermediates of citric acid cycle are also precursors of: - Glucose biosynthesis. - Lipid biosynthesis including fatty acid and cholesterol. Note: Lipid biosynthesis is taken place in cytosol, but the mitochondrial acetyl-CoA (processor) cannot be transported across the inner mitochondrial membrane. Thus, acetylCoA is converted to citrate by ATP-citrate lyase since citrate can cross the membrane. Why citrate synthase is not used? --- Because no ATP is produced. ADP + Pi + oxaloacetate + acetyl-CoA ↔ ATP + citrate + CoA - Amino acid biosynthesis α-ketoglutarate + NAD(P)H + NH4+ ↔ Glu + NAD(P)+ + H2O α-ketoglutarate + Ala ↔ Glu + pyruvate Oxaloacetate + Ala ↔ Asp + pyruvate - Porphyrin biosynthesis - utilizes succinyl-CoA as a starting material. When the citric acid cycle intermediates are transported too much as precursors, the concentration of oxaloacetate is very low. In this case, it is necessary to replenish citric acid cycle intermediates. The main reaction is: - Pyruvate + CO2 + ATP + H2O ↔ oxaloacetate + ADP + Pi The citric acid cycle is truly at the center of metabolism - Reduced products: NADH and FADH2 are reoxidized to produce ATP. - The citric acid intermediates are utilized in the biosynthesis of many vital cellular constituents. 19