Origin of Organic Life
advertisement

abiotic
The Origin of Organic Life
An experiment by Stanley Miller 1953
Archive
Abiotic experiments
In 1828, Friedrich Wohler a German chemist who had studied with Berzelius, attempted to
make an inorganic salt, ammonium cyanate, by mixing solutions of ammonium (NH4+)
and cyanate (CNO-) ions. Wohler was astonished to find that instead of the expected
product, he had made urea, an organic compound present in the urine of animals. Wolher
challenged the then popular vitalists when he wrote, "I must tell you that I can prepare
urea without requiring a kidney or an animal, either man or dog." However, one of the
ingredients used in the synthesis, the cyanate, had been extracted from animal blood, and
the vitalists were not swayed by Wohler's discovery A few years later, Hermann Kolbe, a
student of Wohler's, made the organic compound acetic acid from inorganic substances
that could themselves be prepared directly from pure elements. Decades of laboratory
synthesis produced increasingly complex organic compounds.
In 1953, Stanley Miller, a graduate student at the University of Chicago, helped place this
abiotic (nonliving) synthesis of organic compounds into the context of evolution. Miller
used a laboratory simulation of chemical conditions on the primitive Earth to demonstrate
that the spontaneous synthesis of organic compounds may have been an early stage in the
origin of life.
http://homepages.ihug.com.au/~panopus/abiotic.htm (1 of 7)5/7/2005 10:15:47 AM
abiotic
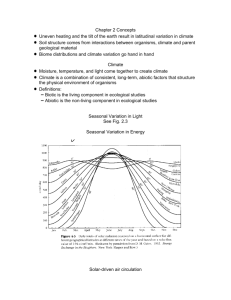
Abiotic synthesis of organic compounds under "early Earth" conditions. Stanley Miller
recreates his 1953 experiment, a laboratory simulation demonstrating that environmental
conditions on the lifeless, primordial Earth favored the synthesis of some organic
molecules. Miller used electrical discharges (simulated lightning) to trigger reactions in a
primitive "atmosphere" of H2O, H2, NH3 (ammonia), and CH4 (methane)-some of the
gases that are belched into the air by volcanoes. From these ingredients Miller's apparatus
made a variety of organic compounds that play key roles in living cells. Similar chemistry
may have set the stage for the origin of life on Earth.
http://homepages.ihug.com.au/~panopus/abiotic.htm (2 of 7)5/7/2005 10:15:47 AM
abiotic
An excerpt from:
Spontaneous generation ...
Despite many arguments, largely theoretical but with some experimental work,
spontaneous generation remained a viable option for the origins of life - for abiogenesis but it was a very confused field. What caused it to change and become focused was the
publication on 23 April 1953 of Crick and Watson's Nature paper on the structure of DNA.
Three weeks later, a graduate student at the University of Chicago named Stanley Miller
published a paper in Science, on 15 May, entitled "A production of amino acids under
http://homepages.ihug.com.au/~panopus/abiotic.htm (3 of 7)5/7/2005 10:15:47 AM
abiotic
possible primitive earth conditions".
Miller was a doctoral student of Nobel laureate Harold C. Urey (a chemist who discovered
deuterium), after he heard a lecture by Urey in which he noted in passing that earth's
primordial hydrogen-rich (reducing) atmosphere would have been favourable for the
formation of simple organic molecules. {Schopf 123} He decided, with Urey's permission,
to test this, assuming an atmosphere of molecular hydrogen (H2), methane (CH4),
ammonia (NH3) and water vapour (H2O). Neither Urey nor Miller knew at this point that
this was in line with Oparin's hypothesis, but as he prepared for the experiments, Miller
read Oparin and mulled it over, along with Urey's hypotheses on the formation of the solar
system. {Schopf 125}
He passed the atmosphere through a glass retort, continuously cycling it for several days,
while exposing it to heat, electrical arcing, and cooling. After two days, the "ocean" (a
flask of water through which the gases were passed) became pale yellow, and on analysis
this turned out to be glycine, the simplest amino acid. They repeated the experiment for a
week, and in the final yellow-brown solution, Miller detected seven amino acids,
including three (glycine, anine and aspartic acid) found in modern living systems. In a
period of three and a half months, Miller had confirmed Urey's and Oparin's hypotheses on
the formation of the precursor molecules of life.
The claim was never that life had been made, but only that the necessary molecules for life
could form spontaneously. Since Wöhler synthesised urea in 1828, this was becoming an
inevitable conclusion - the molecular nature of life was more and more widely accepted
and applied. Now there was no need to think that organic molecules had to come from
organic systems. Later experiments use a more realistic atmosphere, replacing methane
with carbon monoxide or dioxide (CO or CO2), or ammonia with molecular nitrogen
(N2), with similar results.
An alternative to the Oparin-Miller model was proposed by Günter Wächtershäuser, who
suggested that carbon oxides released from deep sea vents could stabilise on ironsulphates, reacting with molecular hydrogen to form organic monomers (simple molecular
units) from which life could form. Others have included the roles of clay substrates as
catalytic templates for molecules to form on before there were genes, the formation of
organic molecules in space (now well-established) seeding the early earth, and a formal
model by Manfred von Eigen of how chemical reactions might generate copies of
themselves - the hypercycle.
http://homepages.ihug.com.au/~panopus/abiotic.htm (4 of 7)5/7/2005 10:15:47 AM
abiotic
Sidney Fox successfully synthesised coascervate "cells" (a coascervate is a mixture of
colloids that can, like lipids in modern cells, form a layer that will enclose molecules, but
which can allow monomers to pass across it). These will, under some conditions, divide as
they "grow" to form new cells.
There has been considerable progress made in recent years - the "Modern origins of life
references" gives citations, but it is a complex and rapidly moving field.
An excerpt from:
EDU_news ...
Study suggests life on Earth sprang from borax minerals January 9, 2004
GAINESVILLE, Fla. --- Researchers at the University of Florida say they have shown that
minerals were key to some of the initial processes that formed life on Earth.
Specifically, a borax-containing mineral known as colemanite helps convert organic
molecules found in interstellar dust clouds into a sugar, known as ribose, central to the
genetic material called RNA. This announcement provides a key step toward solving the 3billion-year-old mystery of how life on Earth began. The findings will appear in Friday's
issue of the journal Science. Steven Benner, Alonso Ricardo, Matthew Carrigan and
Alison Olcott built on a famous experiment done 50 years earlier by Stanley Miller that is
found in many textbooks. In 1953, Miller showed that electric sparks in a primitive
atmosphere made amino acids, the building blocks of proteins.
Miller's experiment failed to identify sugars that were needed for genetic material,
however. "The sugar ribose can be formed from interstellar precursors under prebiotic
conditions," said Benner, who led the research funded by NASA, the National Science
Foundation and The Agouron Institute in Pasadena, Calif. "But ribose is too unstable to
survive under Miller's conditions." Ribose, like most sugars, turns into tar if not handled
carefully. "It is like baking a cake too long," said Benner, a UF distinguished professor of
chemistry and anatomy and cell biology. In 1995, Miller gave up trying to make ribose
prebiotically, writing: "The first genetic material could not have contained ribose or other
sugars because of their instability."
Benner, who also is a member of NASA's Astrobiology Institute, did the first experiments
http://homepages.ihug.com.au/~panopus/abiotic.htm (5 of 7)5/7/2005 10:15:47 AM
abiotic
as an instructor at an international geobiology course last summer funded by the Agouron
Institute and held at the University of Southern California Wrigley Institute for
Environmental Studies. "We asked two questions. First, what simple organic molecules
might have been present on early Earth as starting materials to form ribose? Then, what
might have been present on early Earth to capture ribose and keep it from burning up like
overcooked cake?" Benner said.
To identify simple organic molecules that might be the starting materials, Benner turned to
compounds known to exist in interstellar dust, such as formaldehyde, used to preserve
tissue. "Formaldehyde may not seem to be a good starting point for the life that we know,"
he said. "But it is simple. With only one carbon atom, one oxygen atom and two hydrogen
atoms, there is a lot of formaldehyde to work with in the cosmos."
Benner and his team showed that formaldehyde, with other interstellar compounds, could
form ribose and other sugars when treated in the presence of base materials such as lime, a
material used to adjust the pH level of lawns, among other things. Lime was effective, but
the ribose decomposed soon after it was formed.
Recognizing that ribose had a particular chemical structure that allowed it to bind to
minerals containing the element boron, they turned to another substance called colemanite.
"Colemanite is a mineral containing borate found in Death Valley," he said. "Without it,
ribose turns into a brown tar. With it, ribose and other sugars emerge as clean products."
Benner then showed similar reactions with other borate minerals, including ulexite and
kernite, which is more commonly known as borax.
Benner and his team are the first researchers to succeed in making significant amounts of
ribose under these early conditions.
Joseph Piccirilli, a biological chemist at the Howard Hughes Medical Institute and the
University of Chicago, said Benner's work "has simplicity and brilliance."
"Organic chemists have long known that borate complexes with compounds like ribose,"
Piccirilli said, "and prebiotic scientists have long believed that minerals on the early Earth
played an important role in the origin of life." Until now, "no one has put the two ideas
together," he said.
"We are not claiming that this is how life started," Benner stressed. "We are saying that we
http://homepages.ihug.com.au/~panopus/abiotic.htm (6 of 7)5/7/2005 10:15:47 AM
abiotic
have demonstrated a recipe to make a key part of life without any biochemical machinery.
The more recipes of this type that can be found, the more clues we have about how life
could have actually gotten started on the primitive Earth."
While best classified as basic science, the work has practical biological and medical value.
"Curiously, thinking about how life originated and what form it might take on other
planets helps us design new tools for disease diagnostics and therapy," Benner said.
Diagnostic tools enabled by Benner's work seeking alternative life forms are used today in
the clinic to monitor the load of the viruses that cause AIDS and hepatitis C.
The work also complements other research Benner is conducting that focuses on ancient
forms of life on Earth. In a September report in Nature, Benner and his collaborators
deduced the structure of a protein found in a bacterium that lived several billion years ago
and resurrected the ancient protein. By studying it in the laboratory, the group inferred the
ancient bacteria lived in a hot spring at about 150 degrees Fahrenheit.
With the prebiotic experiments, Benner said, "we are working forward in time, from the
origin of the planet to the first life. With experiments with ancient proteins, we work
backwards in time, from the modern world to the most primitive of bacteria." The group's
goal, he said, is to have the two meet in the middle.
University of Florida
Top
http://homepages.ihug.com.au/~panopus/abiotic.htm (7 of 7)5/7/2005 10:15:47 AM