Electrostatics: Charging Methods & Static Electricity
advertisement

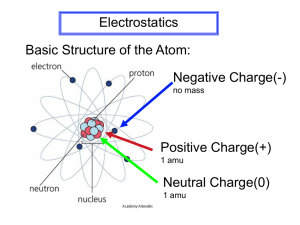
Electrostatics: The study of static electric charges. (Give your experience eg. Combing hair, static cling, sweater over head; then define.) CHARGING FRICTION CONTACT INDUCTION Charge: The net -ve of +ve charge that builds up on an object. Charging: The process of building a net quantity of –ve or +ve charges on an object. Charging by Friction: Transferring electric charges from one substance to another by rubbing. Depending on the materials being used the charges will be different. Eg., acetate when rubbed with silk is positively charged. Ebonite when rubbed with silk is negatively charged. The relative charge is determined by the Electrostatic Series Charging by Contact: Transferring charges from one substance to another by touching. Same charge is received by the material being charged. Charging by Induction: Transferring charges from one substance to another without contact. Opposite charge is received by the material being charged. Reading Assignment: SNC1D1: pp. 460 -501; SNC1P1: pp. 252-273 in your Textbook. 1 The Electrostatic Series – used to predict a charge This can be used to predict what charged will be gained by two objects when they are rubbed together. weak hold on electrons strong hold on electrons acetate glass wool fur or hair silk aluminum cotton paraffin wax ebonite plastic rubber gold When two objects are rubbed together: + • the one closer to the top of the series will lose electrons and become positive • the one closer to the bottom will gain the electrons and become negative - Example: When clothes are put together in the dryer without fabric softener, they will rub against each other and gain static electricity. Suppose cotton socks are dried with a wool sweater. The wool is closer to the top of the electrostatic series, so it has a weak hold on its electrons and will lose them, gaining a positive charge. The cotton is closer to the bottom of the list, so it has a strong hold on electrons, attracting those from the wool. The socks will gain electrons and become negatively charged. The positive wool sweater and the negative cotton socks have opposite charges and so they are attracted to each other. http://www.school- for-champions.com/science/static_materials.htm 2 Mini-quiz to check your understanding 1. What happens to a material that collects electrons on its surface? (a) It has a negative charge (b) It has a positive charge (c) It shoots off sparks 2. Rubbing which materials together would produce the most static electricity? (a) Glass and rubber (b) Cotton and ebonite (c) Silk and aluminum 3. If you combed your hair with a plastic comb, which would give up its electrons? (a) Your hair (b) The comb (c) Your skin, if it was dry If you got all three correct, you are on your way to becoming a champion in science. If you had problems, you had better look over the material again. 3 Charging by Contact On the following diagrams 1) Draw in the location of the negative charges 2) Show the direction of the movement of the negative charges 3) Shade in the position of the moveable leaf 4 Charging by Induction On the following diagrams 1) Draw in the location of the negative charges 2) Show the direction of the movement of the negative charges 3) Shade in the position of the moveable leaf 5 Charging a Pith Ball On the following diagrams; i) Show how the negative and positive charges are arranged and ii) Explain why the pith ball is first attracted then repelled from the rod Discussion 1) The neutral pith ball has equal number of (+) and (-) charges 2) The negative (-) charges on the pith ball are repelled away form the rod. The positive (+) charges on the pith ball are closer to the negative (-) charges on the rod than the pith balls positive (+) charges. The pith balls positive charges are therefore attracted more strongly than the pith balls negative (-) charges are repelled. Hence there is an overall attractio n 3) When rod makes contact, surface negative (-) charges are able to move onto the pith ball, giving it an overall negative charge. 4) The pith ball is then repelled away from the rod. 6 Static Electricity Lab Time: 30 minutes Purpose: You can produce static electricity from many common household materials. In this activity, you can try some combinations of materials to find put which ones produce a static charge. Problem: Which combination of materials results in the strongest electrical charge? Materials: Styrofoam packing chips, Group A Group B Plastic rulers Paper towel Soda Straws Fur Glass rods Cotton Ebonite rods Plastic bag Acetate strips Sandwich wrap Vinyl strips Wool Procedure: 1. Make the following table in your notebook. 2. 3. Choose an object in group A and rub it with a material from group B. 4. Bring the rubbed end close to a small pile of styrofoam packing chips and record the data in the table. 5. Remove the styrofoam packing chips from the rod and hold them in your hand to remove any charge from them. 6. Repeat steps 2-6 for different combinations. 7. When all the combinations of these materials have been tested, indicate in the table whether the charge was strong, fair, poor, not present. 7 Object A Material B # of chips picked up Strength of charge Questions: 1. Of all the combinations you tried, which pair of materials seemed to produce the most static electricity when they were rubbed together? How did you decide this? 2. With which materials were you able to produce a static charge? 3. Why is it necessary to hold the styrofoam packing chips in your hand between each test? 4. If you have experienced any sma ll shocks at home or at school, describe the situation in which they occurred. What pair of materials do you think might be responsible for producing the static electricity? 8 Radioactivity Grounding DISCHARGING Shining light Discharge at a point DISCHARGING: if a charged object has its charge reduced, we say it is discharged or neutralized. When a charged object is discharged by connecting to the ground, we say that the object is grounded. INSULATORS: These are substances in which elections do not move freely from atom to atom. Some good insulators are rubber and plastic. CONDUCTORS: these are substances which allowed electrons to move freely from one atom to another. Some good conductors are copper and aluminum. LIGHTNING RODS: These are placed on the tops of buildings to provide a lowresistance path to ground that can be used to conduct the enormous electrical currents when lightning strikes occur. 9