The role of competitive dominance in the invasive ability of the
advertisement

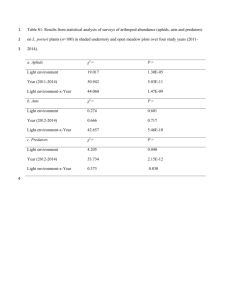
Biol Invasions DOI 10.1007/s10530-007-9103-3 ORIGINAL PAPER The role of competitive dominance in the invasive ability of the Argentine ant (Linepithema humile) Soledad Carpintero Æ Joaquı́n Reyes-López Received: 30 May 2006 / Accepted: 12 February 2007 Springer Science+Business Media B.V. 2007 Abstract To assess the importance of competition in the advance of invasive species, bait stations have been used to determine the dominance hierarchy of a community of native ants in Doñana National Park, southern Spain, and the status of the introduced species Linepithema humile (Argentine ant). Some native species, e.g. Cataglyphis floricola or Camponotus pilicornis, seem to be subordinate, i.e. occupy a low position in the competitive hierarchy; some are dominant (e.g. Pheidole pallidula), and others (e.g. Aphaenogaster senilis) occupy an intermediate position in the hierarchy. The Argentine ant is a competitively dominant species, because of its aggressive behavior and relative abundance. Irrespective of their position in the dominance hierarchy, L. humile and some native species adopt what games theory terms ‘‘the bourgeois strategy’’ during agonistic encounters with other species. Lone workers tend to be submissive in encounters whereas workers in the presence of S. Carpintero Departamento de Ciencias Ambientales, Área de Zoologı́a, Universidad Pablo de Olavide, Ctra. de Utrera km 1, Sevilla 41013, Spain J. Reyes-López (&) Área de Ecologı́a, Campus de Rabanales, Universidad de Córdoba, Cordoba 14071, Spain e-mail: joaquin@uco.es other colony members are aggressive. L. humile was the only species which aggressively displaced large numbers of ants of other species from the bait. L. humile also expanded its range in the course of the experiment, displacing native species from parts of the study area. Keywords Linepithema humile Invasion Dominance hierarchy Bourgeois strategy Introduction Species invasions are a principal component of global change, causing large losses in biodiversity, and economic damage (Arim et al. 2006). Given the importance of this problem, several studies have attempted to understand and predict the success of invasive species, their patterns of spread, and the characteristics of vulnerable invaded ecosystems and communities. Many studies of ants have highlighted the importance of both intraspecific and interspecific competition in structuring communities (Cerdá et al. 1998; Eldridge and Traniello 1981; Fox et al. 1985; Holway 1999; Human and Gordon 1999; Passera et al. 1996; Perfecto 1994; Punttila et al. 1994; Savolainen 1990; Yamaguchi 1992). Competitive ability is important because the resource requirements of many ant species overlap substantially (Pisarski and Vepsäläinen 1989), not 123 Biol Invasions just between closely-related species but also between distantly-related species (Mabelis 1984). As a result, introduction of a new species, for example the invasive Argentine ant (Linepithema humile, Mayr, 1868), may prompt major convulsions in the native ant community (Holway 1999; Human and Gordon 1999; Suarez et al. 1998). An important topic in the study of social behavior is dominance. Dominance can be described as an attribute of any individual or species that usually wins agonistic encounters. The winner is ‘‘dominant’’ and the loser is ‘‘subordinate’’ (De Vries 1998; Drews 1993). Using this definition, several dominance hierarchies have been reported in ant communities (Hölldobler and Wilson 1990; Vepsäläinen and Pisarski 1982). For example, Vepsäläinen and Pisarski (1982) classified species on a scale ranging from those that usually win contests to those that usually lose contests, although this does not necessarily mean that the lowest-ranked species are unable to survive in communities with dominant species. The lowest rank consists of species with very little aggression which usually only fight to defend their nests. Ants of this kind are the same as the ‘‘opportunist’’ species described by Wilson (1971) on bait. The workers of these species are often very efficient at finding new food resources and are often the first to arrive, though they may later be displaced by more aggressive species. Also included in this group are species characterized by small colony and worker sizes and elusive workers that avoid confrontation with other species (Hölldobler and Wilson 1990). The intermediate region of the scale contains species that maintain spatio-temporal territories, defending food sources and their nests (Hölldobler and Lumsden 1980). This type of defense occurs when food or other resources are unpredictable. These species usually come into greater conflict with the dominant species than those in the previous category (Vepsäläinen and Pisarski 1982; Hölldobler and Wilson 1990). Also included in this category are ants that make fairly stable food trails, for example the Old and New World graminivorous ants, and leaf-cutter ants from the tropics (Hölldobler and Lumsden 1980). 123 The highest-ranking species are usually very aggressive, and actively defend a permanent territory containing their nests and a relatively large foraging area. Dominant species usually patrol wide areas and can have an important effect on local communities, not just of ants but also of other arthropods and plants (Hölldobler and Lumsden 1980; Punttila et al. 1994). The dominance hierarchy of these species provides a framework for examining the role of aggressive behavior and interspecific competition in structuring communities. It should be possible to predict the relative abundance of species according to their position in the hierarchy. For example, it is unlikely that two dominant species could co-exist locally, because of their strongly territorial behavior (Holway 1999; Pisarski and Vepsäläinen 1989; Savolainen 1990). Nevertheless, previous studies of social insects have noted a trade-off between interference and exploitative ability in species of differing ranks, which may promote co-existence (Cerdá et al. 1997). Invasive species seem to disrupt this trade-off (Holway 1999; Feener 2000). The Argentine ant has spread beyond its original range to many parts of the world, especially in areas with a Mediterranean climate (both northern and southern latitudes, usually between 30 and 36). Besides being a threat to native ant communities, introduced Argentine ants have been found to cause economic and public health problems (Williams 1994; Vander Meer et al. 1990). Their greatest effect, however, is on the abundance and distribution of native ant species (Fluker and Beardsley 1970; Human and Gordon 1999; Majer 1994; Sanders et al. 2001; Suarez et al. 1998). Their effect on local ant communities is so great because these large colonies of generalists make high demands on a wide range of resources, bringing them into competition with a wide range of species, in a process known as ‘‘diffuse competition’’ (Holway 1999; Suarez et al. 1998). It has even been suggested they become predators of native ant species (Zee and Holway 2006). Several factors have contributed to the spread and establishment of Argentine ant populations (Passera 1994), including their marked aggressiveness toward other species and their capacity Biol Invasions for mass recruitment (Holway et al. 1998; Liang and Silverman 2000; Suarez et al. 1999; Tsutsui et al. 2001). Despite their reputation as an invasive species, surprisingly few field studies have addressed the behavior of Argentine ants, especially in Europe (Fowler et al. 1994; but see Way et al. 1997). To examine competitive interactions between Argentine ants and native ants, and add to existing knowledge of the role of competition in structuring communities, a series of field experiments was designed in Doñana National Park, southern Spain, where the Argentine ant has recently become established. The main objectives of the study were to determine its position in the dominance hierarchy with respect to native species, and to determine how this affects its success as an invader. Experimental Study site Doñana National Park is located on the southwest coast of the Iberian Peninsula (provinces of Huelva and Seville, Spain) and covers an area of approximately 56,544 ha, which includes a large area of marsh at the mouths of the Guadalquivir and Guadiamar rivers. The local climate is ‘‘subhumid’’ Mediterranean, because of the effect of the Atlantic, and, according to Gaussen’s (1968) classification, can be included in the Mediterranean climatic region. The vegetation consists mostly of scrubland, sparsely populated with large isolated cork oaks (Quercus suber) and patches of introduced pines (Pinus pinea). As a result of its rolling topography, a legacy of the sand dune origin of the region, and a shallow water table, the higher areas are characterized by xerophytic vegetation and the lower, damper, areas are characterized by hygrophytic vegetation. A full description of the habitat of Doñana National Park can be found in Carpintero et al. (2003). This study was undertaken in a pinewood next to the Doñana visitors’ center at ‘‘El Acebuche’’. The vegetation in the study area consisted of Mediterranean stone pine (Pinus pinea), and an understorey of Mediterranean scrub mostly comprising Cistus libanotis, Cistus salvifolius, Lygos monosperma, Rosmarinus officinalis, Halimium halimifolium, Lavandula stoechas, Thymus vulgaris, Ulex minor, and Cistus albidus. Methods The Argentine ant completely monopolized the immediate vicinity of the visitors’ center. At the limit of its local range, some distance from the visitors’ center, Argentine ants and native species were both found (Fig. 1). In this area of overlap, six samples of tuna bait in 10 cm · 12 cm plastic trays were placed on a 2 · 3 grid at 4-m intervals. Tuna is often used as bait in studies of interactions between ground-living ants (Andersen and Patel 1994; Human and Gordon 1999; Savolainen and Vepsäläinen 1988; Torres 1984) and in a previous experiment performed in the same area using a variety of bait, tuna bait was shown to attract all species present. Bait is commonly used in studies of agonistic interactions in ants (Cerdá et al. 1998; Holway 1999; Savolainen and Vepsäläinen 1988). Normally, when individual workers of different species meet they move away from each other immediately. Sometimes the more aggressive species may attack but these attacks are usually unsuccessful (Jones and Phillips 1987). When the number of opponents increases, however, for example around high quality food sources, for example bait, the opportunity for escape or evasion decreases, and both intra and interspecific fighting and aggression are common (Cerdá et al. 1998; Holway 1999; Levings and Traniello 1981; Phillips et al. 1986). The work was conducted over three days each in May, July, and September 1990. For each hour of daylight continuous 10-min observations were made of each of the six bait stations. In May and September, observations began at 9:00 am and finished at 8:00 pm. In July observations lasted from 8:00 am to 9:00 pm. During each observation a record was kept of the identity and the number of each ant species on the bait, and of the type of behavioral interactions observed. Each observation was allocated to one of four categories: 123 Biol Invasions Fig. 1 Study site. Pine forest close to the Doñana visitors’ center ‘‘El Acebuche’’, showing the area of overlap of Argentine ants and native species (at a distance of 25–80 m from the center) A only one species seen on the bait; B two or more species present but no aggressive interactions observed; C two or more species on the bait interacting aggressively; and D no ants observed on the bait during the 10min observation period. Behavior during agonistic encounters between species was classified as follows: Aggressive behavior Physical attack: The attacker bites the body, antennae or legs of its antagonist. Chemical attack: The attacker bends its abdomen forward and directs a noxious secretion at its enemy. Threat: The individual faces its opponent with open mandibles. Submissive behavior Escape: The individual moves rapidly away after making contact with another individual. Other behavior Indifference: After making contact, the individual shows neither aggressive nor submissive behavior. 123 Results During the experiment bait was visited by seven ant species. Two of these (Camponotus lateralis and Messor marocanus) were seen very infrequently (n = 3 individuals) and were not included in analyses. All six bait stations were used by the five most abundant species (n = 174 individuals: Linepithema humile, Pheidole pallidula, Tapinoma nigerrima, Aphaenogaster senilis, Cataglyphis floricola), apart from bait station 1 which was never used by T. nigerrima. Colony and worker size, foraging strategy and diet for each of these five species are shown in Table 1. A total of 684, 10-min observations were made of the bait stations. The frequency of each class of observation A–D (Methods section) is shown in Fig. 2. Observations were not distributed evenly across classes (v2 = 515.90, P < 0.001). The most frequent class of observation was type A (only one species seen on the bait) indicating that ants rarely share bait stations. The least frequent class was type B (two or more species present but no aggressive interactions observed), indicating that not only did ants rarely share baits, but also when they did they usually interacted aggressively (72.6% of observations with >1 species and aggressive interactions). There was also a Biol Invasions Table 1 Worker and colony size, type of recruitment and diet of the most abundant species found at the bait stations Species Worker Size (mm) Colony Size Recruitment Diet L. humile (*) P. pallidula (*) T. nigerrima (**) A. senilis (***) C. floricola (****) 2.1–2.6 1.6–2.6 2.8–5.1 6.4–7.7 5.4–6.7 Thousands Thousands Thousands Hundreds Hundreds Mass Mass Mass Group No recruitment Omnivore (honeydew) Omnivore (graminivore) Omnivore (honeydew) Omnivore Petals + insects Terms in parentheses indicate favored food types. Data from (*), Bernard 1968; (**), Cerdá et al. 1998; (***), Gómez and Espadaler 1996; (****), Tinaut 1993 and Cerdá et al. 1996 Class D (n=218) Class A (n=393) Class C (n=53) Class B (n=20) type C, where different species interact aggressively on the bait stations, the mean number of individuals of each species was greater than for type B, suggesting that abundance is important in prompting aggressive interactions. For species with mass recruitment, the mean number of individuals counted during class A observations was significantly different from the mean number counted during class B and class C observations (Mann–Whitney U test, P < 0.005, in all cases). Agonistic interactions between species Fig. 2 Frequencies of observations of each class: A = only one species seen on the bait; B = two or more species with no aggressive interactions; C = two or more species with aggressive interactions; D = no ants seen relatively high frequency of observations of type D (31.9%) when no ants were seen on the bait. This phenomenon has been reported in several studies, which have shown that the borders between the territories of some dominant species constitute a ‘‘no man’s land’’ low in ant abundance and species richness (Adams 1994; Mabelis 1984; Mercier et al. 1997). For each species, the frequency of observations in each class and the mean number of individuals seen on the bait are shown in Table 2. When only one species was observed on the bait (observation class A), those species with mass recruitment (L. humile, P. pallidula, T. nigerrima) were present in relatively large numbers (Table 2). In those observations where two or more species shared the bait but did not interact aggressively (class B) there were few individuals present, even of massrecruiting species. Two or more species can only coexist on a bait station without conflict if very few individuals are present. In observations of Species altered their behavior depending on the number of colony-members present on the bait, and on whether they were initiating or responding to a confrontation (Table 3). During encounters, species tended to initiate attacks if there were more individuals of their own species on the bait, and escape if there were fewer, with the exception of C. floricola, for which aggressive behavior was never observed. Indeed, the numbers of individuals present on bait when encounters resulted in initiation of attack and escape differed significantly (Kruskal–Wallis test: H = 1.82, P = 0.0001, n = 341). A similar abundance pattern was observed for responses to encounters, although differences were not significant (Kruskal–Wallis test: H = 1.22, P = 0.27, n = 422). Individual behavior also depended upon whether the ant was initiating or responding to behavior during an encounter. Species initiating encounters tended to be more aggressive (M-L, v2 = 12.5, df = 4, P = 0.0140) whereas those on the receiving end usually, but not always, behaved submissively (M-L, v2 = 16.84, df = 4, P = 0.0021). This was reflected in the frequencies 123 Biol Invasions Table 2 Frequencies of observations of each class (N) and the associated mean (s.d.) number of individuals on the bait Class A Class B Class C L. humile P. pallidula T. nigerrima A. senilis C. floricola N = 191 36.8 ± 51.1 N = 15 2.3 ± 2.2 N = 39 6.1 ± 13.1 N = 54 39.3 ± 44.8 N = 11 2.7 ± 1.6 N = 32 20.8 ± 33.3 N = 28 30.7 ± 31.6 N=4 1.0 ± 0.0 N=9 2.8 ± 1.9 N = 11 2.0 ± 2.0 N=8 1.5 ± 0.7 N = 26 5.4 ± 7.3 N = 108 1.9 ± 1.7 N=6 1.5 ± 0.5 N=7 2.6 ± 2.9 Class A = only one species seen on the bait; B = two or more species with no aggressive interactions; C = two or more species with aggressive interactions Table 3 Frequency of encounters (N) classified by behavior type (aggression: physical attack, chemical attack and threat; submission: escape) of individuals initiating and L. humile P. pallidula T. nigerrima Aggression (action) Aggression (response) Submission (response) Submission (action) 38.7 ± 43.8 N = 124 58.6 ± 37.6 N = 55 3.2 ± 3.1 N=8 8.3 ± 8.8 N=9 65 ± 49.5 N=2 1 N=1 3.5 ± 0.7 N=2 1±0 N=2 1±0 N=2 N=0 12.6 ± 8.6 N = 148 N=0 3.6 ± 1.5 N=8 8.6 ± 16.4 N = 69 14.6 ± 18.4 N = 146 1.4 ± 0.6 N = 69 2.6 ± 2.5 N = 17 2.5 ± 1.6 N = 93 C. floricola A. senilis of observations of initiation and response associated with aggression or escape. The frequency of encounters involving aggressive behavior (physical attack, chemical attack, and threat) was 98.24% for individuals initiating encounters (actors) (n = 341) compared with only 4.83% for individuals responding to encounters (receivers) (n = 414). The frequency of encounters involving submissive behavior (escapes) was 1.76% for actors (n = 341) and 95.17% for receivers (n = 414). Whether an individual acted as an actor or receiver depended on the number of conspecifics on the bait, because for all species the mean number of conspecifics present for actors was greater than for receivers (30.8 ± 40.3 (s.d.) compared with 8.0 ± 4.5; Kruskal–Wallis test: H = 221.9, P = 0, n = 902). The most common form of aggressive behavior observed for all species was physical attack, which accounted for 90.98% of all aggressive encounters. The only species observed to carry out chemical attacks was L. humile when confronted by T. nigerrima, but this occurred much less frequently than physical attacks (five occasions 123 responding to encounters, with the mean number of conspecifics ± s.d. on the bait at the time of encounter N=0 N=0 only). The only submissive behavior observed was escape. Only ten fatal attacks were observed: on four occasions A. senilis killed L. humile workers, and on six occasions the same species killed P. pallidula workers. Displacements of ant species from bait Bait stations were not always occupied by the same species, and replacement of one species on a bait station by another reflected daily or seasonal activity patterns (unpublished data) and the outcome of conflicts. For example, during the cooler times of day, in the morning and late afternoon, bait was occupied by L. humile, P. pallidula, T. nigerrima, or A. senilis whereas during the hottest period, around mid-day, it was occupied by the thermophilous C. floricola (Tinaut 1993). Such patterns of dual bait occupancy do not suggest aggressive displacement. On other occasions, however, a species took over a bait station initially occupied by another species after an aggressive encounter. The only species recorded to take over a bait station Biol Invasions already occupied by a large number of workers of another species was the Argentine ant (on two occasions they displaced P. pallidula and on one occasion A. senilis). Although A. senilis, T. nigerrima, and P. pallidula sometimes initiated fights and attempted to take food from bait stations already occupied by large numbers of another species, they were never observed to succeed in displacing their opponents. In one of the two cases of aggressive displacement of P. pallidula by L. humile, which occurred in July, the Argentine ant was observed to form a broad trail from the bait to the nest of the displaced P. pallidula within an hour of arrival. In this instance P. pallidula did not put up much aggressive resistance. Not only did the Argentine ants succeed in occupying the bait station, they also entered the nest of P. pallidula and, shortly afterward, workers of the Argentine ant were seen removing larvae of the native species, thus confirming brood predation (as suggested by Zee and Holway 2006). The following day, P. pallidula had completely disappeared from the area and only workers of L. humile were seen entering and leaving the nest. In the second instance of aggressive displacement of P. pallidula by L. humile, this time in September, the same situation was observed, with complete displacement of P. pallidula from the bait then occupation of its nest. In contrast, on two occasions when skirmishes were observed between relatively small numbers of L. humile and P. pallidula workers at bait stations, it was P. pallidula rather than the Argentine ant that gained control of the bait station. On the occasion of aggressive displacement of A. senilis by L. humile six individual workers of A. senilis showing very aggressive behavior were seen on the bait, suggesting recruitment to the bait station. Despite this, Argentine ants moved on to the bait and, although A. senilis killed some workers of the introduced species, they eventually had to surrender the bait station. On two other occasions, however, A. senilis was seen to successfully defend bait stations against mass-recruiting species—once they held the bait station against approximately forty P. pallidula workers trying to take food; on another occasion they actively defended the bait against a similar number of L. humile workers. Seasonal patterns in the activity of different species (unpublished data) might also have affected the occupancy of bait stations; such patterns could explain, for example, why T. nigerrima was seen only in May. But longterm species displacement patterns were observed that cannot be accounted for by seasonal activity. For example, during the last month of sampling, L. humile was the only species found at some bait stations, suggesting the introduced species had expanded its range (Fig. 3). Discussion Ant species present in the study area rarely shared bait stations. When two or more species were present on the same bait encounters between workers were generally antagonistic (Fig. 2); it was thus possible to develop a dominance hierarchy for the species present. C. floricola never behaved aggressively and always fled from bait stations in the presence of other species (Table 3) and so was placed at the bottom of the hierarchy. The level of aggression shown by a species is often related to its diet and social structure; species living in small colonies, for example, tend to be less aggressive than those forming very large colonies. Interference competition is most likely to be observed for species exploiting large and/or predictably-located food resources (Holway 1999; Levings and Traniello 100 80 60 40 20 0 MAY JULY C. floricola A. senilis P. pallidula L. humile SEPTEMBER T. nigerrima Fig. 3 Percentage of monthly bait-station observations associated with the occupancy of each species (n = 684) 123 Biol Invasions 1981; Phillips et al. 1986). The diet of C. floricola consists largely of small dead insects randomly distributed in the environment, typically harvested by solitary foragers. Unusually for its genus, C. floricola gathers large numbers of Halimium halimifolium petals which also provide a limited, seasonal food resource, thus further promoting a non-aggressive ‘‘scramble’’ harvesting strategy (Cerdá et al. 1996). The colonies of this species are small, so it would be risky and expensive to be aggressive towards neighbors and defend a territory (Levings and Traniello 1981). The other native species occupy the next level in the hierarchy and actively defend food sources and their nests, leading to territories that change in size and shape over time (Hölldobler and Lumsden 1980; Hölldobler and Wilson 1990). All these species recruit conspecifics to exploit and defend food resources. P. pallidula and T. nigerrima recruit many workers (>100 ants) to food sources (mass recruitment), leading to the formation of food trails (Detrain et al. 1991), whereas A. senilis recruits workers in smaller numbers (<100 ants, group recruitment) (Cerdá et al. 1988; Gómez and Espadaler 1996). The first species to occupy a bait station in large numbers maintains its ownership of the bait station. There were no instances of a bait station held by large numbers of workers of one native species being taken over by another. For these species many factors contribute to obtaining and defending resources, including efficiency and speed in locating new food resources, distance to the nest, colony size, and recruitment efficiency (Eldridge and Traniello 1981). The observation that the first group or individual to occupy a resource will hold it against competitors has been reported for other animal species, including other ant species (Maynard Smith and Parker 1976; Torres 1984). Describing this finding, Hölldobler and Wilson (1990) quoted a remark made by General Nathan Bedford Forrest during the American Civil War: ‘‘The winners arrive on the battlefield first with the greatest numbers.’’ When these species become owners of a bait station their levels of aggression increase. These species are relatively submissive when they do not have ownership of the bait, however (Table 3), although individuals will try to steal bait from occupied stations. 123 The Argentine ant maintains larger fixed territories, not just to monopolize particular food sources but also to control the entire foraging area by excluding all other ant species (Fluker and Beardsley 1970; Human and Gordon 1999; Sanders et al. 2001; Wilson 1971). The territoriality of the Argentine ant is similar to that of other dominant species (Adams 1994; Gordon 1995; Hölldobler and Wilson 1990; Traniello and Levins 1986)—several nests are scattered throughout the territory rather than concentrated in the center. Groups of workers with queens and/ or young can be found in all areas of the territory. This nest arrangement enables a large proportion of the area encompassed within these large territories to be efficiently patrolled and exploited while simultaneously avoiding the high cost of with transporting food from distant locations to a single central nest. The Argentine ant has even been observed to move its young after finding a new food source (Holway and Case 2000). This system has important implications for optimum territory size—if the cost of territory defense is proportional to perimeter length, and benefits are proportional to territory area, then territories should be as large as possible (i.e. maximizing the perimeter-to-area ratio). Indeed, in polygynous and polycalic species, for example L. humile, territory sizes are limited only by the availability of suitable habitat (Hölldobler and Lumsden 1980; Holway and Case 2000). In the area bordering the range of the introduced ant, both L. humile and native species (except C. floricola) altered their behavior depending upon the number of conspecific workers present on the bait. This suggests that L. humile, P. pallidula, T. nigerrima, and A. senilis adopt the ‘‘bourgeois strategy’’ (Maynard Smith and Parker 1976; Rasmusen 1989), behaving both as ‘‘hawks’’ (aggressive behavior) and as ‘‘doves’’ (submissive behavior) depending on ownership of the bait. This pattern of increased aggression triggered by the presence of large numbers of conspecifics associated with ownership of a food resource or proximity to a nest has also been observed in other ant species (Cammaerts and Cammaerts 1996; Le Moli et al. 1984; Mabelis 1984; Mayades et al. 1993; Mercier et al. 1997; Wenseleers et al. 2002). The mechanisms Biol Invasions responsible for changing aggressive behavior were not examined here, but other studies, for example that of Cammaerts and Cammaerts (1996) on P. pallidula, found that workers use pheromones to identify shifting territories. Another study has described A. senilis as a subordinate species with little aggression (Cerdá et al. 1998). Here, by contrast, when a sufficient number of workers were present this species fiercely defended bait stations and was the only species that actually killed workers of other species while defending bait. When few conspecific workers were present and the bait was held by another species, however, they never behaved aggressively and always fled after encounters with other species. Individual workers of A. senilis were observed to snatch pieces of bait and then retreat rapidly. This behavior has been observed in other species of Aphaenogaster (A. rudis) and has been called the ‘‘jackal strategy’’ (Lynch et al. 1980). The Argentine ant was the only species capable of aggressively taking control of bait stations already wholly occupied by another species. The occupancy of bait stations by the Argentine ant also increased over time (Fig. 3). This observation is not consistent with any seasonal activity patterns, but rather suggests displacement of native ants from the study area by this exotic species. The idea of competitive displacement is supported by two cases in which Argentine ants were seen to expel P. pallidula colonies from their nests after forcing them from baits. It is not known whether these colonies were destroyed or just displaced, as reported in other studies of this and other Pheidole species (Detrain and Pasteels 1992; Fluker and Beardsley 1970). In any case, the Argentine ant has a substantial negative effect on the fitness of competitors, by forcing antagonists to waste time and energy escaping and rebuilding nests and by forcing displaced colonies to risk competing with other colonies of the same or other species (Gordon 1995). Although the competitive ‘‘bourgeois’’ strategy of individual Argentine ants on bait stations is used by several species, the ability of the Argentine ant to form large multi-nest colonies (Hee et al. 2000; Holway 1999; Human and Gordon 1999; Tsutsui et al. 2001) seems to give them the edge when competing for resources (bait) and territories. According to several authors, aggressive behavior is an important component of dominance, because it often results in the establishment of a status enabling privileged access to resources such as food or space. Levels of ant aggression are determined by their position in the hierarchy and by worker density. In general, the more aggressive and the greater the dynamic density (individuals dm–2 min–1) of a colony, the higher the position of the colony in the dominance hierarchy (Savolainen and Vepsäläinen 1988). As noted in other studies of invasive species (Hook and Porter 1990, Dame and Petren 2006), this emphasizes the importance of considering behavioral mechanisms when trying to understand the success of aliens in native ecosystems. Despite the negative effects of this species on ecosystems, few studies have addressed the effect and distribution of the Argentine ant in Spanish protected natural spaces (Carpintero et al. 2003; Carpintero et al. 2005). There is currently no concrete program for the future monitoring and management of this invasive species in Doñana National Park. Acknowledgments The authors would to thank Antonio Priego and Rafa ‘‘el francés’’ for their assistance in the field. David Williams and Ricardo Reques provided many of the bibliographical references, and Alberto Tinaut cleared up all uncertainties about species identification. Our thanks to Carmen Ortega, Alfonso Carpintero, Sylvia Williams, and all the staff and colleagues at Doñana for their help and kindness. We would like also to thank the anonymous referees for valuable comments and suggestions. This work was supported by a grant from the Comunidad Autónoma de Andalucı́a to S. Carpintero. The experiments comply with current legislation in Spain, where they were performed. References Adams ES (1994) Territory defense by the ant Azteca trigona: maintenance of an arboreal ant mosaic. Oecologia 97:202–208 Andersen AN, Patel AD (1994) Meat ants as dominant members of Australian ant communities: an experimental test of their influence on the foraging success and forager abundance of other species. Oecologia 98:15–24 Arim M, Abades SR, Neill PE, Lima M, Marquet PA (2006) Spread dynamics of invasive species. Proc Nat Acad Sci USA 103(2):374–378 123 Biol Invasions Bernard F (1968) Les fourmis (Hymenoptera Formicidae) d’Europe occidentale et septentrionale. Faune de l’Europe et du Bassin Méditerranéen. Ed. Masson. Paris Cammaerts MC, Cammaerts R (1996) Area marking in the ant Pheidole pallidula. Behav Processes 37:21–30 Carpintero S, Reyes-López J, Arias de Reyna L (2003) Impact of human dwellings on the distribution of the exotic ant Argentine ant: a case study in the Doñana National Park, Spain. Biol Conserv 115:279–289 Carpintero S, Reyes-López J, Arias de Reyna L (2005) Impact of Argentine ants Linepithema humile) on an arboreal ant community in Doñana National Park, Spain. Biodivers Conserv 14:151–163 Cerdá X, Retana J, Carpintero S, Cros S (1996) An unusual ant diet: Cataglyphis floricola feeding on petals. Insectes Soc 43:101–104 Cerdá X, Retana J, Cros S (1997) Thermal disruption of transitive hierarchies in Mediterranean ant communities. J Animal Ecol 66:363–374 Cerdá X, Retana J, Cros S (1998) Critical thermal limits in Mediterranean ant species: trade-off between mortality risk and foraging performance. Funct Ecol 12:45–55 Dame A, Petren K (2006) Behavioural mechanisms of invasion and displacement in Pacific island geckos (Hemidactylus). Animal Behav (71):1165–1173 Detrain C, Deneubourg JL, Goss S, Quinet Y (1991) Dynamics of collective exploration in the ant Pheidole pallidula. Psyche 98(1):21–31 Detrain C, Pasteels JM (1992) Caste polyethism and collective defense in the ant Pheidole pallidula: the outcome of quantitative differences in recruitment. Behav Ecol Sociobiol 29:405–412 DeVries H (1998) Finding a dominance order most consistent with a linear hierarchy: a new procedure and review. Animal Behav 55:827–843 Drews C (1993) The concept and definition of dominance in animal behaviour. Behaviour 125(3–4):283–290 Eldridge SA, Traniello FA (1981) Chemical interference competition by Monomorium minimum (Hymenoptera: Formicidae). Oecologia 51:265–270 Feener DH (2000) Is the assembly of ant communities mediated by parasitoids? Oikos 90:79–88 Fluker SS, Beardsley JW (1970) Sympatric associations of three ants: Iridomyrmex humilis, Pheidole megacephala and Anoplolepis longipes in Hawaii. Ann Entomol Soc Am 63:1290–1296 Fowler HG, Schlindwein MN, Medeiros MA (1994) Exotic ants and community simplification in Brazil: a review of the impact of exotic ants on native ant assemblages. In: Williams DF (ed) Exotic ants. Biology, Impact, and control of introduced species. Westview Press, Boulder, CO, pp 151–162 Fox BJ, Fox MD, Archer E (1985) Experimental confirmation of competition between two dominant species of Iridomyrmex (Hym.: Formicidae). Aust J Ecol 10:105–110 Gaussen H (1968) Les indices sérothermique et hygrotermique en Péninsule Hispanique et en Africa du Nord partie N.W. Collectanea Botán 7:499–504 123 Gómez C, Espadaler X (1996) Distancias de forrajeo, áreas de forrajeo y distribución espacial de Aphaenogaster senilis Mayr (Hym. Formicidae). Miscellánia Zool 19(2):19–25 Gordon DH (1995) The development of an ant colonýs foraging range. Animal Behav 49:649–659 Hee JJ, Holway DA, Suarez AV, Case TJ (2000) Role of propagule size in the success of incipient colonies of the invasive Argentine ant. Conserv Biol 14(2):559– 563 Hölldobler B, Lumsden CJ (1980) Territorial strategies in ants. Science 210:732–739 Hölldobler B, Wilson EO (1990) The ants. Springer-Verlag, Berlin Holway DA (1999) Competitive mechanisms underlying the displacement of native ants by the invasive argentine ant. Ecology 80(1):238–251 Holway DA, Case TJ (2000) Mechanisms of dispersed central-place foraging in polydomous colonies of the Argentine ant. Animal Behav 59:433–441 Holway DA, Suarez AV, Case TJ (1998) Loss of intraspecific aggression in the success of a widespread invasive social insect. Science 282:949–952 Hook AW, Porter SD (1990) Destruction of harvester ant colonies by invading fire ants in south-central Texas (Hym.: Formicidae). Southwest Nat 35:477–478 Human KG, Gordon DM (1999) Behavioral interactions of the invasive Argentine ant with native ant species. Insectes Soc 46:159–163 Jones SR, Phillips SA (1987) Aggressive and defensive propensities of Solenopsis invicta (Hym.: Formicidae) and three indigenous ant species in Texas. Tex J Sci 39(2):107–115 Le Moli F, Mori A, Parmigiani S (1984). Studies on interspecific aggression among red wood ant species. Formica rufa L. vs Formica lugubris Zett. (Hym.: Formicidae). Monitore Zool Ital (N.S.) 18:41–51 Levings SC, Traniello JFA (1981) Territoriality, nest dispersion, and community structure in ants. Psyche 88:265–319 Liang D, Silverman J (2000) ‘‘You are what you eat’’: Diet modifies cuticular hydrocarbons and nestmate recognition in the Argentine ant, Linepithema humile. Naturwissenschaften 87:412–416 Lynch JF, Balinsky EC, Vail SG (1980) Foraging patterns in three sympatric forest ant species, Prenolepis imparis, Paratrechina melanderi and Aphaenogaster rudis (Hymenoptera: Formicidae). Ecol Entomol 5:353–371 Mabelis AA (1984) Interference between Wood ants and other ant species (Hymenoptera, Formicidae). Neth J Zool 34(1):1–20 Majer JD (1994) Spread of Argentine ants (Linepithema humile), with special reference to western Australia. In: Williams DF (ed) Exotic ants. Biology, impact, and control of introduced species. Westview Press, Oxford, pp 161–173 Mayade S, Cammaerts M-C, Suzzoni JP (1993) Homerange marking and territorial marking in Cataglyphis cursor (Hymenoptera, Formicidae). Behav Processes 30(2):131–142 Biol Invasions Maynard Smith J, Parker GA (1976) The logic of asymmetric contests. Animal Behav 24:159–175 Mercier JL, Lenoir A, Dejean A (1997) Ritualised versus aggressive behaviours displayed by Polyrhachis laboriosa (F. Smith) during intraspecific competition. Behav Processes 41:39–50 Passera L (1994) Characteristics of tramp species. In: Williams DF (ed) Exotic ants. Biology, impact, and control of introduced species. Westview Press, Oxford, pp 23–43 Passera L, Roncim E, Kaufmann B, Keller L (1996) Increased soldier production in ant colonies exposed to intraspecific competition. Nature 379:630–631 Perfecto I (1994) Foraging behavior as a determinant of asymmetric competitive interaction between two ant species in a tropical agroecosystem. Oecologia 98:184–192 Phillips SA, Jones SR, Claborn DM (1986) Temporal foraging patterns of Solenopsis invicta and native ants of Central Texas. In: Lofgren CS, Vander Meer RK (eds) Fire ants and leafcutting ants: biology and management. Westview Press, Boulder, CO Pisarski B, Vepsäläinen K (1989) Competition hierarchies in ant communities (Hym., Formicidae). Ann Zool Warszawa 42(13):321–328 Punttila P, Haila Y, Niemelä J, Pajunen T (1994) Ant communities in fragments of old-growth taiga and managed surroundings. Ann Zool Fennici 31:131–144 Rasmusen E (1989) Games and information. An introduction to Game Theory. Blackwell ed., Oxford Sanders NJ, Barton KE, Gordon DM (2001) Long-term dynamics of the distribution of the invasive Argentine ant, Linepithema humile, and native ant taxa in northern California. Oecologia 127:123–130 Savolainen R (1990) Colony success of the submissive ant Formica fusca within territories of the dominant Formica polyctena. Ecological Entomology 15:79–85 Savolainen R, Vepsäläinen K (1988) A competition hierarchy among boreal ants: impact on resource partitioning and community structure. Oikos 51:135– 155 Suarez AV, Bolger DT, Case TJ (1998) Effects of fragmentation and invasion on native ant communities in coastal Southern California. Ecology 79(6):2041– 2056 Suarez AV, Tsutsui ND, Holway DA, Case TJ (1999) Behavioral and genetic differentiation between native and introduced populations of the Argentine ant. Biol Invas 1:1–11 Tinaut A (1993) Cataglyphis floricola nov. sp. new species for the genus Cataglyphis Förster, 1850 (Hymenoptera, Formicidae) in the Iberian Peninsula. Bull Soc Entomol Suisse 66:123–134 Torres JA (1984) Niches and coexistence of ant communities in Puerto Rico: repeated patterns. Biotropica 16(4):284–295 Traniello JFA, Levings SC (1986) Intra- and intercolony patterns of nest dispersion in the ant Lasius neoniger: correlations with territoriality and foraging ecology. Oecologia 69:413–419 Tsutsui ND, Suarez AV, Holway DA, Case TJ (2001) Relationships among native and introduced populations of the Argentine ant (Linepithema humile) and the source of introduced populations. Mol Ecol 10:2151–2161 Vander Meer RK, Jaffe K, Cedeno A (eds) (1990) Applied myrmecology. A world perspective. Westview Studies in Insect Biology, Boulder, San Francisco and Oxford Vepsäläinen K, Pisarski B (1982) Assembly of island ant communities. Ann Zool Fennici 19:327–335 Way MJ, Cammell ME, Paiva MR, Collingwood CA (1997) Distribution and dynamics of the Argentine ant Linepithema (Iridomyrmex) humile (Mayr) in relation to vegetation, soil conditions, topography and native competitor ants in Portugal. Insec Soc 44(4):415–433 Wenseleers T, Billen J, Hefetz A (2002) Territorial marking in the desert ant Cataglyphis niger: does it pay to play Bourgeois? J Ins Behav 15(1):85–93 Williams DF (ed) (1994) Exotic ants. Biology, impact, and control of introduced species. Westview Press, Oxford Wilson EO (1971) The insect societies. Harvard University Press. Cambridge Yamaguchi T (1992) Interspecific interference for nest sites between Leptothorax congruus and Monomorium intrudens. Ins Soc 39:117–127 Zee J, Holway D (2006) Nest raiding by the invasive Argentine ant on colonies of the harvester ant, Pogonomyrmex subnitidus. Insec Soc 53:161–167 123