Lecture 1
advertisement

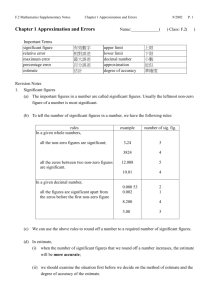
Chemistry 102D Dr. Amy Nicely 205 Chem Annex anicely@illinois.edu Office Hours: Mondays 3-4pm Thursdays 1-2pm or by appointment Required Materials: Chemistry, 8th ed., Zumdahl i>clicker Scientific calculator Grading Policy: On-line Homework Class Participation Quizzes (10 x 10) Hour Exams (3 x 100) Final Exam 70 pts. 30 pts. 100 pts. 300 pts. 300 pts. 800 pts. No make-up quizzes or exams On-line Homework: 13 Homework assignments on Lon-Capa due dates in the on-line Syllabus and calendar credit for all problems completed by deadline normalized to 70 points Course Homepage http:// www.chem.uiuc.edu Course Web Sites Chem 102D Lon-Capa = net i.d. AD password Class Participation: Purchase and register an i>clicker Bring i>clicker to lecture every day Receive 1 point for every problem attempted and 0.2 bonus points for every correct answer normalized to 30 points Quiz scores: weekly quizzes - material from Lecture and Quiz section each quiz worth 10 points - keep 10 highest 100 points Exams: Hour exams take place on Thursday evenings from 7:00-8:10pm Exam dates are September 23, October 28 and December 2 Conflict exams are offered at 5:35pm on the same dates; you must sign up in advance 100 points each Exams: Final exam is Friday, December 17 from 1:30 - 4:30pm The exam is cumulative 300 points Grades 800 points total 93 - 100% 90 – 92.9% 87 – 89.9% 83 – 86.9% 80 – 82.9% etc., etc., etc. (744 - 800) = A (720 - 743) = A(696 - 719) = B+ (664 - 695) = B (640 - 663) = B- observation measurement of physical phenomena number units charge of an electron - 0.00000000000000000160 coulombs Scientific Notation 0.000000000000000000160 integer number x 10n 1-9 0 1.60 x 10 = 1.60 1.60 x 101 = 16.0 1.60 x 10-1 = 0.160 -19 1.60 x 10 1.60 or 1.6 or 1.600 Significant figures uncertainty in last digit 5.08 x 104 3 sig.fig. 5.07 5.09 50,700 50,900 5.0800 x 104 5 sig.fig 5.0799 5.0801 50,799 50,801 1. All non zero digits are significant 2. Zeros between digits are significant 3. Zeros to left of digits are not significant 4. Zeros to right of digits and decimals are significant Significant figures (47.03) x (0.00540) = 0.253962 = 0.254 4 sig.fig 3 sig.fig -3 (5.40 x 10 ) 3 sig.fig rounding limiting round at end of ≥ 5 round up < 5 round down calculations 45.6 1 decimal place 0.369 3 decimal places + 83.51 2 decimal places 129.47900 129.5 limiting Significant figures 732.11 + 6.3 = 760.00 do addition (subtraction) first 732.11+ 6.3 = 738.41 (738.4) 2 decimal 1 decimal 1 decimal do multiplication (division) last 738.4 4 sig fig 738.41 = 0.971592105 4 sig fig 760.00 5 sig fig 760.00 0.9716 SI units mass kilogram length meter time second temperature kelvin amount mole TK = TC + 273.15 mole = 6.02 x 1023 kg m s K mol SI units mass length time temperature amount kilogram meter second kelvin mole kg m s K mol volume = length3 1 liter (L) = (1 dm)3 = (10 cm)3 = 1000 cm3 1 cm3 = 1 mL Dimensional Analysis 1. Start with data given, including units. 2. Determine the units in the answer 3. Find conversion factors What is the mass, in grams, of 1.00 gallon of water? The density of water is 1 g/mL. 3 ∞ 1.00 gal 4 qts 1 gal 1 gal = 4 qt ∞ 1L 1.057 qt 1.057 qt = 1 L ∞ 1000 mL 1L 1 g = 1 mL ∞ 1 g = 3784.295 g 1 mL 3780 g 3.78 x 103 g 1000 mL = 1 L