Biol 2122 Exam #4 Reminder: The following study guide is exactly
advertisement

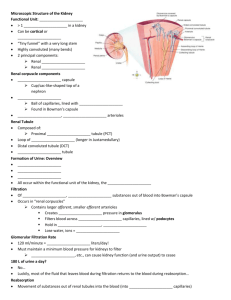
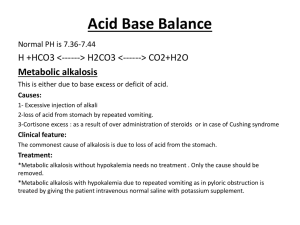
Biol 2122 Exam #4 Reminder: The following study guide is exactly that, a guide. Use it to direct your studies for the third exam. The text should be used to clarify any questions you have. You are still responsible for all class notes covered or not covered in my lectures. Good luck to you all. Urinary System: 1- Describe the functions of the kidneys given in lecture 2- Identify the major components (gross anatomy) of the urinary system. 3- The kidneys are located in a ___________________________ position. 4- The _____________ kidney is lower than the ____________. Why? 5- What structures exit the renal hilus? 6- What tissues make up the connective tissue layer of the external kidney? 7- Identify all arterial and venous branches within the kidneys 8- What comprises the renal corpuscle? 9- The functional unit of the kidney is the __________________. 10- The three types of cells found in the renal corpuscle are the: 11- Which one makes rennin? Responds to concentrations and rate flow? Phagocytic properties? 12- Describe the events, in order, that occur in the renin –angiotensin system. 13- What does aldosterone do? 14- Identify the major parts of the nephron tubular system. - proximal convoluted tubule loop of Henle (ascending and descending loop) distal convoluted tubule collecting duct 15- The collecting ducts are located in the renal ________________. 16- Describe what occurs during the three phases of renal physiology 17- What is the glomerular filtration rate (GFR)? 18- ________% of the filtrate is reabsorbed. 19The descending limb of the loop of Henle is impermeable to _____________ and permeable to ___________________. 20The ascending limb of the loop of Henle is impermeable to _____________ and permeable to ___________________. 21- What is the trigone of the bladder? 22- At about __________ cc of urine, the signal is sent to the brain for micturition. 23- What are the normal components of urine? Abnormal components of urine? Fluid, acid-base and electrolyes 1- What is the normal arterial blood pH? 2What conditions can cause metabolic acidosis? Metabolic alkalosis? Respiratory acidosis? Respiratory alkalosis? 3Computing the anion gap can show there is relative balance between cations and anions. Be able to compute an anion gap. What is hyperchloremic acidosis? 4- How does the Na+ - H+ antiport system work? 5pH? How can hyperaldosteronism affect this system? What will happen to blood 6- How does chronic diarrhea affect blood pH? Vomiting? 7- What is the breakdown of the 60% body weight composition of water? 8- Distinguish between an electrolyte and non-electrolyte 9- Obligatory water loss includes: 10- Understand the flow chart concerning regulation of water intake, water output, regulation of sodium by aldosterone, ANH and baroceptors. 11. What happens during dehydration? Hypotonic hydration? 12- Aldosterone acts upon the distal convoluted tubule and collecting ducts for Na+ retention 13- Amount of water reabsorbed in the distal segments of the kidney tubules is proportional to ADH release (increase in ADH secretion = increase in water resorption 14- ANH: Reduces blood pressure and blood volume by inhibiting nearly all events that promote vasoconstriction and sodium and water retention. 15 K+: Regulation of Potassium Balance Potassium is the chief intracellular cation Relative intracellular-extracellular potassium concentrations directly affects a cell's resting membrane potential, therefore a slight change on either side of the membrane has profound effects (ie. on neurons and muscle fibers) Potassium is part of the body's buffer system, which resists changes in pH of body fluids; ECF potassium levels rise with acidosis (decrease pH) as potassium leave cells and fall with alkalosis (increase pH) as potassium moves into cells. 16. Increase Na+ in plasma= decreased K+ in plasma; vice-versa for filtrate in kidney. 17. What is the effect of PTH and calcitonin on blood calcium, phosphate and magnesium? 18. What is a buffer? 19. The three types of chemical buffers in the body are 20 Bicarbonate buffers: Major extracellular buffering system HCO3- functions as a weak base while H2CO3 functions as a weak acid. 21. Acids are proton donors and bases are proton acceptors. 22. Two examples of physiological buffers are: Endocrine and reproduction given out in class (see attached word doc. notes on schedule)