LA9. Measurement and Graphing
advertisement
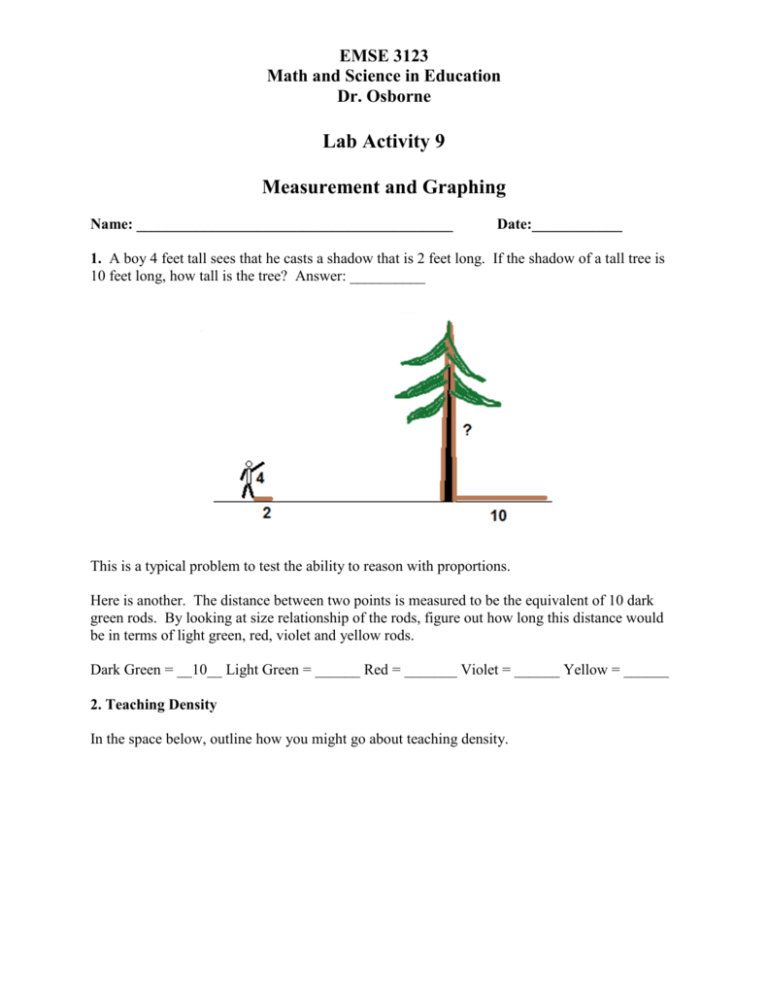
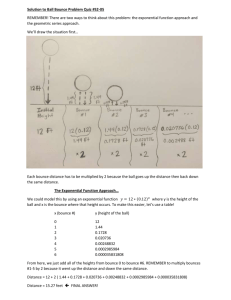
EMSE 3123 Math and Science in Education Dr. Osborne Lab Activity 9 Measurement and Graphing Name: __________________________________________ Date:____________ 1. A boy 4 feet tall sees that he casts a shadow that is 2 feet long. If the shadow of a tall tree is 10 feet long, how tall is the tree? Answer: __________ This is a typical problem to test the ability to reason with proportions. Here is another. The distance between two points is measured to be the equivalent of 10 dark green rods. By looking at size relationship of the rods, figure out how long this distance would be in terms of light green, red, violet and yellow rods. Dark Green = __10__ Light Green = ______ Red = _______ Violet = ______ Yellow = ______ 2. Teaching Density In the space below, outline how you might go about teaching density. State the general formula for density. Based on your formula, answer the questions below. a. The box below weighs 30 grams. What is its density?______________ Will it float or sink? _______________ How much would 100 cc of this material weigh? ________________ How many cubes would you need to have 50 grams? _____________ Study cans of diet coke and regular coke. Predict whether each would float or sink. Determine the density of each by mass and displacement of water. Complete the table below. Product Diet Coke Mass Volume Density Regular Coke Explain what causes the difference in density that you observed. Explain this activity from the standpoint of the Learning Cycle Approach. Which parts correspond to Exploration,, Concept Formation, and Concept Application. 3. Analytical and Graphic Skills A very basic question in many science investigations is “How does one thing depend on another?” Each of these “things” is called a variable since we can change or vary its value. An important skill in math and science is the ability to analyze the relationship between variables. Sometimes these variables can be very complicated but in this chapter we will look at some simple relationships. Finding a relationship between two variables means first identifying the variables, then performing a “controlled” experiment to see how changing one variable affects the other. A controlled experiment involves being able to “neutralize’ all except the two variables you are investigating. Eventually you wish to come up with a mathematical relationship (equation) between the two variables. This often will involve producing a graph showing the relationship. Height and Bounce Drop a ball from various heights and for each height, collect data to measure the height that the ball bounces back. Place your data in a data table and see if you can find a mathematical relationship between height dropped and height of bounce. The two variables in this activity are height of drop and height of bounce. The variable that you have direct control over is called the independent variable. In this investigation it is the height of drop. The height of bounce depends on this variable and is called the dependent variable. The two tables below represent some data obtained by different groups of students. How do you find a mathematical relationship between the height dropped and the height of bounce? If you study the data in Table A closely, you will probably notice that the height of drop is 3 times the height of bounce. The data in Table B are more likely to be the kind that your students will find. Here, the relationship is not so obvious and we need to use graphical analysis to find the relationship. The Bouncing Ball Investigation Collect data in the table below to produce a Bounce vs Dropped graph. Questions and Analysis 1. What is the relationship (equation) connecting height dropped to height of bounce for your data? 2. What is the slope of the graph? 3. What would be the height of bounce if the ball was dropped from a height of 75 cm? 4. From what height would you need to drop the ball in order to get a bounce of 35 cm? 5. Using your equation, find the height of bounce if the height of drop was 1000 cm. 4. Joseph Black and the discovery of the Heat of Fusion. Joseph Black was professor of Chemistry and Medicine at the University of Edinburgh. In 1761 he studied ice and snow melting on the nearby hills in the Spring. He noticed that heat energy of the Sun was probably involved. This also led to the discovery of the Heat of Vaporization. Prior to his discoveries, whiskey distillers had no idea how much wood or coal was needed to boil water to make steam. Setting up the investigation. • • • • • • Two identical cans are filled with equal amounts of water. One can is frozen the other has a temperature of 0°C. A thermometer is placed in each can (it is in the can on the right before freezing) The liquid warms up to room temperature. The frozen water absorbs the same amount of heat but the temperature does not increase. There is heat of fusion. Heat is absorbed when ice melts. Heat is given off when water freezes. On the next page is a table of data actually collected during one of these investigations. Data table: Plot these data on the graph below. The values for Time are indicated on the x-axis already for you. 1. Indicate the temperatures on the y-axis and plot the data correctly on the graph. Note that the times of the measurements may or may not coincide with the vertical time lines. 2. After three hours, how many degrees of heat were absorbed by the water? 3. If the water absorbed this much heat, the ice must also have absorbed the same amount of heat. What happened to the heat absorbed by the ice? 4. Joseph Black used the term “latent heat.” What does this term mean? 5. We have measured what happens when ice warms up. What happens when water boils?