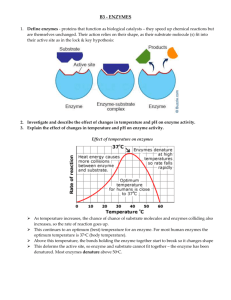
2.3 Entrapping Enzymes
advertisement

Food Biotechnology 2 December 2003 333-535 A Dr. B. Lee ENZYME TECHNOLOGY IN FOOD PROCESSING Word & PowerPoint files available at http://f.rival13.free.fr/enzymes Lydia ETCHEBEST 260101578 Frédéric RIVAL 260098444 Enzymes technology in food processing Dr B. Lee Table of Contents 1. Introduction 1.1 Enzymes types and sources 1.2 Reaction conditions 1.3 Commercialization of enzymes processes 2. Immobilization of enzymes 2.1 Carrier Binding 2.1.1 Physical Adsorption mode 2.1.2 Ionic Binding mode 2.1.3 Covalent Binding mode 2.2 Cross Linking 2.3 Entrapping Enzymes 3. Industrial application 3.1 Cheese making 3.2 Meat tenderization 3.3 Bread making 3.4 Production of beverages and fruit juices 3.5 Starch and sugar industries 3.6 The modification of fats and oils 3.7 Other food enzymes from GMOs 4. Legal and safety implications 5. Protein engineering of enzymes 6. Future prospects 7. References ________________________________________________________________________________ Lydia ETCHEBEST & Frédéric RIVAL 1 Enzymes technology in food processing Dr B. Lee 1. Introduction The use of enzymes to accomplish specific desirable changes in food has been practiced for centuries. A few examples of the ancient use include the use of malted barley for starch conversions in brewing beer and wrapping the meat in the bruised leaves of the papaya tree to tenderize it. Since then, mankind has tried to improve these processes for fast growing food industry and in order to satisfy consumer demands for healthy and natural, tasty, consistent, convenient and low cost products. Often these processes were passed along from generation to generation by trial and error. Later, more systematic approaches have been used. An enzyme is a protein that speeds up (catalyses) chemical reactions in physiological processes in the body (e g digestion) and industrial applications for food products (e.g. fermentation of wine and curdling of cheese). An enzyme acts as a catalyst, regulating the rate of chemical reaction taking place without itself being altered at the end of the process. Enzymes are used in food production, rather than other chemical modifications, because enzymatic reactions are carried out under mild temperature and pH conditions and are highly specific. They have also been used as they can help to reduce processing costs, increase yield and improve product handling, shelf-life and sensory characteristics with modifying raw materials and aiding in the processing or cooking stages. The roles of enzymes include: enhancement of flavour and aroma, removal of unwanted flavours and taints, enhancement of digestibility, modification of texture to aid processing and final product appearance, upgrading raw materials. The main enzyme activity utilised in food processing applications is protease. However, applications utilising lipases and carbohydrate degrading activities are also becoming widespread. The first enzyme to be isolated was diastase(an amylase) from malt in 1833 and the enzyme activity in soy flour was patented as a bleaching agent in 1934. Now there are companies who produce enzyme preparations specifically for baking. Other enzymes which may be used more in the future. There is, however, different legislation covering the use of enzymes in food, in different countries. There are also controversial issues with respect to labeling of products. Should added enzymes be regarded as processing aids, and if their activity is destroyed during processing be omitted from labeling or are they additives which need to be recorded on the labels? For many years researches were carried out on the actions of enzymes, particularly those that cause food deterioration, such as pectic enzymes that cause destruction and separation of pectic substances in citrus juice and polyphenol oxidase that cause browning, off-flavor development and loss of vitamins in fruits and vegetables. Until recently, chemical and food engineers have gained considerable interest in the potential usefulness of enzymes in processing. Since 1950s, tremendous progress has been made in the development of the submerged fermentation techniques and this has reduced the cost and increased the availability of industrial enzymes. In the early 1970s, advances in the technology of immobilized enzymes systems became of significance in the processing of foods and the production of food and chemical ingredients. This ________________________________________________________________________________ Lydia ETCHEBEST & Frédéric RIVAL 2 Enzymes technology in food processing Dr B. Lee technique allows for much greater utilization of the relatively expensive enzyme; such as glucose isomerase in the production of high-fructose corn syrup from starch by fixation of the protein onto the column through which a continuous stream of the liquefied starch slurry could be passed (Roller and Goodenough, 1999). In the late 1980s, the food enzyme, chymosin, derived from genetically modified organisms (GMO5) was first introduced in the markets. The large expansion in the application of enzymes in the manufacturing and processing industries has largely been in two areas: the enhancement of traditional processes and the development of totally new uses based on an understanding of enzyme properties (Chaplin and Bucke, 1990). Nowadays, the food industry is the second largest user of enzymes (the detergent industry is the first). “The challenge to food technologists is to recognize the potential of biotechnology to fulfill the food requirements of today’s society” (Sanders, 1991). 1.1 Enzymes types and sources Biologically active enzymes may be extracted from any living organism: animal, plant and microbiological sources. Most organisms have certain ‘core’ enzymes in common. For instance, enzymes of the Embden-Meyerhof pathway can be found in microbes, plants and animals. Similarly, amylase activity is found widely in human saliva, in plant seedling and in many microbes that use starch as an energy source. For enzymes like these, there are many potential sources. Other enzymes are specific to an organism or even provide that organism its characteristic features. Examples are the specialized enzyme systems in nitrogen-fixing bacteria and enzyme alliinase in onion and related plants, which catalyses the breakdown of peptide precursor to liberate sulphur-containing volatiles that defines the characteristic aroma. In cases like these, the source is limited as well as obvious. Although some animal and plant enzymes such as rennet and papain, are still in use, the large majority of bulk enzymes today are manufactured using fungal or bacterial fermentation. The advantages of using sources from fungi and bacteria have is that they can be easily grown and are usually not difficult to scale up a production process. In addition, the sources are not subject to seasonal or other factors, e.g. chymosin (rennin) that is extracted from the fourth stomachs of young calves for cheese production. The majority of enzymes that have so far been used are hydrolytic enzymes and many of these are produced extracelluarly by fungi. In general, animals and plants are poor sources of enzymes as they are slow growing and expensive. Extraction of enzymes from animal tissues or plants cells can also be difficult and time consuming, further increases the production cost of the enzymes. The possibility of producing larger quantities of enzyme from animal and plant sources by use of tissue culture methods is now being explored. Some proteins, such as vaccines, are already being produced in tissue culture. With microbial enzymes it is often possible to increase the yields by changes in the growth conditions, addition of inducers, or strain selection, including increasing the number of gene copies by genetic engineering (Price, 1999). With enzymes from animal and plant sources, the yields may be increased by the introduction of the appropriate genes and their promoter regions into the more rapidly growing microorganisms. This has removed the technical problems of ________________________________________________________________________________ Lydia ETCHEBEST & Frédéric RIVAL 3 Enzymes technology in food processing Dr B. Lee securing adequate sources of raw materials. However, there are often problems, such as the formation of inclusion bodies through incorrect folding, the lack of glycosylation, or degradation of the recombinant protein, which have to be overcome before a satisfactory product is obtained. There are also strict controls on the use of recombinant protein in the food industry. Types of enzyme The process of traditional products like cheese is due to enzymes that are endogenous; that is they occur naturally in the tissues of plant or animal or in the micro-organism. The endogenous enzymes can be manipulated to some extent to improve product quality but there are limitations. The idea of adding enzymes from other sources (exogenous enzymes), to improve existing reactions or to initiate new reactions, dates from the start of this century (Wolnak, 1980). Early work in the USA led to development of enzymes for the leather industry and started the commercial production of papain for use in the beer industry. Enzymes are often referred to as endogenous or exogenous in foodstuff. Exogenous enzymes are added during processing to involve a wide range of effects. Among these are the control of texture, appearance and nutritive value, as well as the generation of desirable flavors and aromas or their precursors. The applications of these enzymes to generate a desirable end product are often a blanching act in which the degree of enzymatic modification of foodstuff must be carefully controlled. For example, the use of proteases in hydrolyzing proteins, such as that from soybean can cause production of bitter peptides if hydrolysis proceeds too far. One way of eliminating such bitter peptides is by treatment with peptidases to yield a protein-like material plastein. Endogenous enzymes may cause desirable or deleterious effects in texture, aroma, flavor and appearance just as exogenous enzymes. For example, natural food flavors such as terpenes, hydrocarbons, alcohols, aldehydes, ketones, esters, lactones, amines, and sulfur-containing compounds are enzymatically produced in fruits and vegetables. The major difference is that endogenous enzymes are already in the foodstuff and control may be more difficult. 1.2 Reaction conditions Enzymes have a number of distinct advantages over conventional chemical catalysts. Primarily are their specificity and selectivity, not only for particular reactions, but also in their discrimination between similar parts of molecules (regiospecificity) or between optical isomers (stereospecificity). This means that the chosen reaction can be catalyzed to the exclusion of side reactions, eliminating undesirable by-products. Thus, higher productivities may be achieved, reducing material costs. As a bonus, the product is generated in an uncontaminated state, so reducing purification costs and the downstream environmental burden. Enzymes work under generally mild processing conditions of temperature, pressure and PH. Classical enzyme studies, are carried out in dilute aqueous solutions under optimal conditions with only substrate, enzyme, buffer and necessary co-factor present. The efficiency of the reaction is measured by the enzyme activity, which was defined by the International Union of Biochemistry in the 1 960s in an attempt to produce a standard system. One Unit (U) is defined as ________________________________________________________________________________ Lydia ETCHEBEST & Frédéric RIVAL 4 Enzymes technology in food processing Dr B. Lee “the amount of enzyme that catalyses the transformation of 1 of substrate per minute under defined conditions”. The defined conditions normally refer to 25°C and optimal substrate concentration and pH; however, these conditions are rarely found when enzymes are used in the food industry (Fullbrook, 1983) and it is difficult to predict activity and therefore the amount of enzyme that is required. Fullbrook also raises difficult questions about how a mole of industrial substrate, e.g. corn starch, can be defined when the molecular weight varies and the fact that enzyme activity in industry may be measured, not in terms of µmoles of substrate transformed but in terms of reduced viscosity or a related chemical value, e.g. a color standard. Although there is a great deal of published information about enzymes, the application of these data to the industrial context is not always straightforward. Other problems in applying pure biochemical criteria to the food situation are associated with substrate concentration, which is rarely optimal and normally governed by other factors such as solubility. The optimum temperature of commercial enzymes (typically around 50 to 100°C) is also far removed from the standard temperature of 25°C and the pH optimum may be temperature dependent (Fullbrook, 1983). Physical factors also affect the enzymes and there are certainly differences between reaction rates in aqueous solution and when enzymes are membrane bound. When enzymes are immobilized or encapsulated for convenience in food processing, the properties of the enzymes will also change, Reactions at low water activity or in fat/water mixtures (e.g. in the modifications of lipids where the lipid/water interface is important) are also outside the classical enzyme studies. Application of these reactions bas been hindered by a lack of understanding of the basic chemistry, although the enzymatic modification of lipids has considerable commercial potential (Critchley, 1987). There are many food enzymes available that originate from different sources and therefore have different pH and temperature characteristics. It is worth testing a number of these to see if there are significant differences in performance or not. In the case of proteases, there is a wide range available with p11 optima from 2.5 to 9 although they do have different affmities for certain amino-acid bonds. Other types of enzymes generally have narrower ranges of optimum pH. Recent advances in genetic engineering have provided the means for improving the stability of enzymes; this is achieved by altering the structure at vulnerable points by substitution of a different amino acid. Another factor that may limit the usefulness of an enzyme in the industrial context is product inhibition. In normal metabolism, this property is useful, as it helps regulate metabolic pathways, but if the enzyme is required for the complete conversion of a substrate, the product needs to be removed to increase the percentage conversion. Enzyme processes need to be designed so that the desired changes can occur. The product may be removed to increase conversion and the design of enzyme reactors is critical. When enzymes are used over relatively long periods and at elevated temperature, there is a decline in enzyme activity. In some applications, this is welcome, as active enzyme may be unacceptable in the final food product. In other applications, it leads to decreased conversion rate and loss of efficiency. Again, design of the process can overcome these problems so that a constant degree of conversion is achieved. ________________________________________________________________________________ Lydia ETCHEBEST & Frédéric RIVAL 5 Enzymes technology in food processing Dr B. Lee 1.3 Commercialization of enzymes processes When enzymes are considered for use in a food process, it is essential to ensure that they will confer some commercial benefit. There are several ways of defining this latter parameter. Enzymes may improve the conversion of a raw material to its constituent parts as in the hydrolysis of starch to glucose. Acid hydrolysis gives limited conversion, whereas enzymes can improve the yield. This example of starch hydrolysis also illustrates another beneficial effect of using enzymes, namely that the effluent from enzyme hydrolysis is less toxic and therefore cheaper in terms of waste disposal. In the brewing industry, savings on raw material costs can be achieved by the use of enzymes in the mashing process. The traditional mash process relies on the enzyme activities in the malt constituent to hydrolyze the macromolecules of malt and barley into fermentable substrate. Malt is an expensive commodity, however, and it is also variable in terms of enzyme activity. Since brewing is a complex process, the complete replacement of malt enzymes by commercial enzymes may have other effects on the quality of the final product. Rather than total replacement of malt enzymes, commercial enzymes are often used in conjunction with the malt enzymes, so that brewers can standardize the processes and produce consistent quality beer, regardless of raw material fluctuations. Thus the commercial benefits of using enzymes may be expressed in different ways as: - improved conversion - an environmental benefit - cost savings on raw material - standardization of the process. Given the fact that food is biological in nature and that food processing involves some type of conversion of raw materials to processed foods, it is surprising that enzymes are so little used in the industry. In their book Food Biotechnology, Angold et al. (1989) presented several reasons why biotechnology (which includes enzyme technology) has not found greater use in the food industry. They first differentiate between small-scale and large-scale biotechnology. The pharmaceutical industry is typical example of the small-scale operation where the high costs of research and development can be recouped by charging (relative) high prices for drugs. Indeed, it could be argued that pharmaceutical research and development creates markets, as without research into diseases and ailments, no cures could be found. In contrast, the food industry can be described as “large-scale, commodity transformation characterized by a low margin operation” (Angold et al., 1989). Since food is a basic commodity, consumers expect it to be available at a reasonable cost. Moreover, it is difficult to improve food significantly so that is might attract a premium price. People in the Western World will pay a little extra for improved quality but, apart from specialties like caviar or truffles, food generally is cheap. In addition, the population in developed countries already consumes a sufficient variety and quantity of food to satisfy their nutritional requirements and over-consumption is now recognized as undesirable. There is therefore a limit to the market size and expansion can only be achieved by increasing the market share of a particular company. Food is a traditional, craft-based industry and consumers are already suspicious about scientists “messing about” with their food. The public has seen so many contradictory statements by food experts, that the credibility of science as a whole has decreased. Improvements in the processing of ________________________________________________________________________________ Lydia ETCHEBEST & Frédéric RIVAL 6 Enzymes technology in food processing Dr B. Lee food are more likely to be achieved through optimization of existing processes or through advances in engineering to allow efficient production of novel products (e.g. co extrusion machines). The economic system of the consumer and producer is normally allowed to find its own balanced in the so-called free market, but food is such an important strategic commodity, that there is sometimes political intervention. The exogenous enzymes play a major role in industries such as food & beverages, starch sugar & alcohol, animal feed, brewing, textiles, paper & pulp, leather, detergents and health care. Many companies produce enzymes for food application e.g. biocone or biocatalyst. The groups of these different enzymes are: Amylases Amylases act on starch (amylose and amylopectin). They split starch into dextrins and sugars by cleaving the a-1,4 glycosidic linkages in the interior of the starch chain. Amylases can be derived from bacteria and fungi. They play a major role in the animal feed, health care, detergents, food & beverages, brewing, textiles, starch, sugar & alcohol industries. Amyloglucosidase Also called glucoamylase, this enzyme acts on starch, dextrins and sugars by cleaving the a-1, 4 glycosidic linkages releasing stepwise from the end of the chain. It is widely used in the manufacture of glucose and for conversion of carbohydrates to fermentable sugars. They play a major role in starch sugar & alcohol industries. Cellulase Cellulase acts on cellulose molecules by hydrolysing the b-1,4 glycosidic linkages. It largely produces cellobiose, which can ultimately yield glucose units, depending on the characteristic of the enzyme. Cellulases find wide application in the food & beverages and textiles industries. Catalase Catalase is the enzyme that breaks down hydrogen peroxide to water and molecular oxygen. Mainly used in the textiles industry, catalase effectively removes the residual hydrogen peroxide, ensuring that the fabric is peroxide-free. Lipase Lipase breaks down natural lipids and oils to free fatty acids and glycerol. This group of enzymes is widely used in the leather and detergents industries. Glucanases Glucanase act on b-1,3 and b-1,4 bonds in b-D-glucans. B-glucanases are of particular interest to the brewing industry, where they act on the glucans that impede clarification of wort and filtration of beer. This enzyme is also widely used in the animal feed industry. Hemicellulase Hemicellulases act on hemicellulose (also called pentosan), a polymer of pentose sugars. They are mainly used in the baking (food & beverages) industry to improve the quality of dough, the ________________________________________________________________________________ Lydia ETCHEBEST & Frédéric RIVAL 7 Enzymes technology in food processing Dr B. Lee softness of the crumb and volume. They are also used in the animal feed and the pharmaceutical industries. Phytase Phytase dephosphorylates phytin. By converting phytin phosphorus into an available form, it helps reduce the quantity of supplemented phosphorus. Phytase is recommended for use in the animal feed industry for monogastric animals on a plant diet. Protease Proteases are enzymes that act on proteins and convert them to peptides and free amino acids. They play an important role in the food & beverages, detergents and leather industries. Depending on the application acid, neutral and alkaline proteases are available. Pectinase This enzyme group has a heterogeneous collection of several activities in varying proportion. Pectinases act on pectins and their derivatives and play a major role in the food & beverages industry. Rennet Rennet, which is biologically prepared from the Mucor strain, is used as a milk coagulant in the preparation of cheese. The food & beverages industry, specifically the dairy industry, has accepted microbial rennet as the next best thing to natural rennet. Tannase Tannase is an esterase-based formulation that hydrolyses the acyl esters of tannins, making tannins more soluble at a lower temperature and pH. They are used in the production of instant tea in the food & beverages industry. Xylanase Xylanases cleave chains of b-1,4-xylosidic linkages in Xylans. They are mainly used in the food & beverages and paper & pulp industries. However, each group has several enzymes and the names depend on the company. Each enzyme has a specificity to improve the process. The most important advantage is the regularity of production thanks to exogenous enzymes. The quality of endogenous enzymes is very variable. The tables show the different amylases for baking products that the company: Biocone produce. ________________________________________________________________________________ Lydia ETCHEBEST & Frédéric RIVAL 8 Enzymes technology in food processing Dr B. Lee Name Principle activities Application notes Additional data & information Amylase A011P Amylase Fungal alpha-amylase, protease free, full range of activities to 120,000 SKB. Increases oven spring by over 15% compared to control. Contains no protease, which allows greater amylase dose range to be beneficial. Catamyl Plus Mixed Amylases, Pentosanases Fungal and bacterial product Non GMO alternative. to prevent staling in bread. Depol 150L Amylase Liquid bacterial product for French style bread. Depol 371P Maltase/AMG Increases level of available Suitable for use in rye bread and particularly good in low sugar doughs. glucose Depol 414P Alpha-Amylase Speciality amylase for French type bread. Depol 680P Mixed Amylase, AMG Improves structure and and Pentosanase volume of rye bread. Can be used to increase shelf-life. Beneficial in the production of malt loaf. Enhanced volume in French type bread. Gives more desirable crust colour. 2. Methods of Immobilization When immobilizing an enzyme to a surface, it is most important to choose a method of attachment that will prevent loss of enzyme activity by not changing the chemical nature or reactive groups in the binding site of the enzyme. In other words, attach the enzyme but do as little damage as possible. It is desired to avoid reaction with the essential binding site group of the enzyme. Alternatively, an active site can be protected during attachment as long as the protective groups can be removed later on without loss of enzyme activity. In some cases, this protective function can be fulfilled by a substrate or a competitive inhibitor of the enzyme. The surface on which the enzyme is immobilized is responsible for retaining the structure in the enzyme through hydrogen bonding or the formation of electron transition complexes. These links will prevent vibration of the enzyme and thus increase thermal stability. The micro environment of surface and enzyme has a charged nature that can cause a shift in the optimum pH of the enzyme of up to 2 pH units. This may be accompanied by a general broadening of the pH region in which the enzyme can work effectively, allowing enzymes that normally do not have similar pH regions to work together. Carrier-Binding : the binding of enzymes to water-insoluble carriers Cross-Linking: intermolecular cross-linking of enzymes by bi-functional or multifunctional reagents. Entrapping : incorporating enzymes into the lattices of a semi-permeable gel or enclosing the enzymes in a semi-permeable polymer membrane ________________________________________________________________________________ Lydia ETCHEBEST & Frédéric RIVAL 9 Enzymes technology in food processing Dr B. Lee 2.1 Carrier-Binding: The carrier-binding method is the oldest immobilization technique for enzymes. In this method, the amount of enzyme bound to the carrier and the activity after immobilization depend on the nature of the carrier. The following picture shows how the enzyme is bound to the carrier: The selection of the carrier depends on the nature of the enzyme itself, as well as the: - Particle size - Surface area - Molar ratio of hydrophilic to hydrophobic groups - Chemical composition In general, an increase in the ratio of hydrophilic groups and in the concentration of bound enzymes, results in a higher activity of the immobilized enzymes. Some of the most commonly used carriers for enzyme immobilization are polysaccharide derivatives such as cellulose, dextran, agarose, and polyacrylamide gel. According to the binding mode of the enzyme, the carrier-binding method can be further subclassified into: Physical Adsorption Ionic Binding Covalent Binding 2.1.1 Physical Adsorption Mode: This method for the immobilization of an enzyme is based on the physical adsorption of enzyme protein on the surface of water-insoluble carriers. Hence, the method causes little or no conformational change of the enzyme or destruction of its active center. If a suitable carrier is found, this method can be both simple and cheap. However, it has the disadvantage that the adsorbed enzyme may leak from the carrier during use due to a weak binding force between the enzyme and the carrier. The earliest example of enzyme immobilization using this method is the adsorption of beta-D-fructo-furanosidase onto aluminum hydroxide. The processes available for physical adsorption of enzymes are: ________________________________________________________________________________ Lydia ETCHEBEST & Frédéric RIVAL 10 Enzymes technology in food processing Dr B. Lee - Static Procedure - Electro-deposition - Reactor Loading Process - Mixing or Shaking Bath Loading A major advantage of adsorption as a general method of immobilizing enzymes is that usually no reagents and only a minimum of activation steps are required. Adsorption tends to be less disruptive to the enzymatic protein than chemical means of attachment because the binding is mainly by hydrogen bonds, multiple salt linkages, and Van der Waal's forces. In this respect, the method bears the greatest similarity to the situation found in natural biological membranes and has been used to model such systems. Because of the weak bonds involved, desorption of the protein resulting from changes in temperature, pH, ionic strength or even the mere presence of substrate, is often observed. Another disadvantage is non-specific, further adsorption of other proteins or other substances as the immobilized enzyme is used. This may alter the properties of the immobilized enzyme or, if the substance adsorbed is a substrate for the enzyme, the rate will probably decrease depending on the surface mobility of enzyme and substrate. Adsorption of the enzyme may be necessary to facilitate the covalent reactions. Stabilization of enzymes temporarily adsorbed onto a matrix has been achieved by cross-linking the protein in a chemical reaction subsequent to its physical adsorption. 2.1.2 Ionic Binding Mode: The ionic binding method relies on the ionic binding of the enzyme protein to water-insoluble carriers containing ion-exchange residues. Polysaccharides and synthetic polymers having ion-exchange centers are usually used as carriers. The binding of an enzyme to the carrier is easily carried out, and the conditions are much milder than those needed for the covalent binding method. Hence, the ionic binding method causes little changes in the conformation and the active site of the enzyme. Therefore, this method yields immobilized enzymes with high activity in most cases. Leakage of enzymes from the carrier may occur in substrate solutions of high ionic strength or upon variation of pH. This is because the binding forces between enzyme proteins and carriers are weaker than those in covalent binding. The main difference between ionic binding and physical adsorption is that the enzyme to carrier linkages is much stronger for ionic binding although weaker than in covalent binding. ________________________________________________________________________________ Lydia ETCHEBEST & Frédéric RIVAL 11 Enzymes technology in food processing Dr B. Lee 2.1.3 Covalent Binding Mode: The most intensely studied of the immobilization techniques is the formation of covalent bonds between the enzyme and the support matrix. When trying to select the type of reaction by which a given protein should be immobilized, the choice is limited by two characteristics: (1) the binding reaction must be performed under conditions that do not cause loss of enzymatic activity, and (2) the active site of the enzyme must be unaffected by the reagents used. The covalent binding method is based on the binding of enzymes and water-insoluble carriers by covalent bonds. The functional groups that may take part in this binding are listed below: Amino group Hydroxyl group Thiol group Carboxyl group Imidazole group Threonine group Sulfhydryl group, Phenolic group Indole group This method can be further classified into diazo, peptide and alkylation methods according to the mode of linkage. The conditions for immobilization by covalent binding are much more complicated and less mild than in the cases of physical adsorption and ionic binding. Therefore, covalent binding may alter the conformational structure and active center of the enzyme, resulting in major loss of activity and/or changes of the substrate. However, the binding force between enzyme and carrier is so strong that no leakage of the enzymes occurs, even in the presence of substrate or solution of high ionic strength. Covalent attachment to a support matrix must involve only functional groups of the enzyme that are not essential for catalytic action. Higher activities result from prevention of inactivation reactions with amino acid residues of the active sites. A number of protective methods have been devised: Covalent attachment of the enzyme in the presence of a competitive inhibitor or substrate. A reversible, covalently linked enzyme-inhibitor complex. A chemically modified soluble enzyme whose covalent linkage to the matrix is achieved by newly incorporated residues. A zymogen precursor. The active site of the enzyme must not be hindered. There must be ample space between the enzyme and the backbone. It is possible in some cases to increase the number of reactive residues of an enzyme in order to increase the yield of the immobilized enzyme. This provides alternative reaction sites to those essential for enzymatic activity. As with cross-linking, covalent bonding should provide stable, immobilized enzyme derivatives that do not leach enzyme into the surrounding solution. The wide variety of binding reactions and insoluble carriers (with functional groups capable of covalent coupling or being activated to give such groups) makes this a generally applicable method of immobilization. This is true even if very little is known about the protein structure or active site of the enzyme to be coupled. ________________________________________________________________________________ Lydia ETCHEBEST & Frédéric RIVAL 12 Enzymes technology in food processing Dr B. Lee 2.2 Cross-Linking Immobilization of enzymes has been achieved by intermolecular cross-linking of the protein, either to other protein molecules or to functional groups on an insoluble support matrix. Cross-linking an enzyme to itself is both expensive and insufficient, as some of the protein material will inevitably be acting mainly as a support. This will result in relatively low enzymatic activity. Generally, cross-linking is best used in conjunction with one of the other methods. It is used mostly as a means of stabilizing adsorbed enzymes and also for preventing leakage from polyacrylamide gels. Since the enzyme is covalently linked to the support matrix, very little desorption is likely using this method. Marshall (1973), for example, reported that carbamy phosphokinase crosslinked to alkyl amine glass with glutaraldehyde lost only 16% of its activity after continuous use in a column at room temperature for fourteen days. The most common reagent used for cross-linking is glutaraldehyde. Cross-linking reactions are carried out under relatively severe conditions. These harsh conditions can change the conformation of active center of the enzyme; and so may lead to significant loss of activity. 2.3 Entrapping Enzymes The entrapment method of immobilization is based on the localization of an enzyme within the lattice of a polymer matrix or membrane. It is done in such a way as to retain protein while allowing penetration of substrate. It can be classified into lattice and micro capsule types. ________________________________________________________________________________ Lydia ETCHEBEST & Frédéric RIVAL 13 Enzymes technology in food processing Dr B. Lee This method differs from the covalent binding and cross linking in that the enzyme itself does not bind to the gel matrix or membrane. This results in a wide applicability. The conditions used in the chemical polymerization reaction are relatively severe and result in the loss of enzyme activity. Therefore, careful selection of the most suitable conditions for the immobilization of various enzymes is required. Lattice-Type entrapment involves entrapping enzymes within the interstitial spaces of a cross-linked water-insoluble polymer. Some synthetic polymers such as polyacrylamide, polyvinylalcohol, etc... and natural polymer (starch) have been used to immobilize enzymes using this technique. Microcapsule-Type entrapping involves enclosing the enzymes within semi permeable polymer membranes. The preparation of enzyme micro capsules requires extremely wellcontrolled conditions and the procedures for micro capsulation of enzymes can be classified as: Interfacial Polymerization Method: In this procedure, enzymes are enclosed in semi permeable membranes of polymers. An aqueous mixture of the enzyme and hydrophilic monomer are emulsified in a water-immiscible organic solvent. Then the same hydrophilic monomer is added to the organic solvent by stirring. Polymerization of the monomers then occurs at the interface between the aqueous and organic solvent phases in the emulsion. The result is that the enzyme in the aqueous phase is enclosed in a membrane of polymer. Liquid Drying: In this process, a polymer is dissolved in a water-immiscible organic solvent which has a boiling point lower than that of water. An aqueous solution of enzyme is dispersed in the organic phase to form a first emulsion of water-in-oil type. The first emulsion containing aqueous micro droplets is then dispersed in an aqueous phase containing protective colloidal substances such as gelatin, and surfactants, and a secondary emulsion is prepared. The organic solvent in then removed by warming in vacuum. A polymer membrane is thus produced to give enzyme micro capsules. Phase Separation: One purification method for polymers involves dissolving the polymer in an organic solvent and re-precipitating it. This is accomplished by adding another organic solvent which is miscible with the first, but which does not dissolve the polymer. ________________________________________________________________________________ Lydia ETCHEBEST & Frédéric RIVAL 14 Enzymes technology in food processing Dr B. Lee The form of an immobilized enzyme can be classified into four types: particles, membranes, tubes, and fibers. Most immobilized enzymes are in particle form for ease of handling and ease of application. Particles - The particle form is described in the above section. Membranes - Enzyme membranes can be prepared by attaching enzymes to membranetype carriers, or by molding into membrane form. The molding is done after the enzymes have been enclosed within semi-permeate membranes of polymer by entrapment. Tubes - Enzyme tubes are produced using Nylon and polyacrylamide tubes as carriers. The polymer tube is first treated in a series of chemical reactions and the enzyme is bound by diazo coupling to give a tube in a final step. Fibers - Enzymes that have been immobilized by entrapment in fibers to form enzyme fibers. The solid supports used for enzyme immobilization can be inorganic or organic. Some organic supports include: Polysaccharides, Proteins, Carbon, Polystyrenes, Polyacrylates, Maleic Anhydride based Copolymers, Polypeptides, Vinyl and Allyl Polymers, and Polyamides. 3. Industrial applications 3.1 Cheese making According to legend, cheese was discovered thousands of years B.C. by a traveller who placed milk into a pouch made from a sheep stomach. During the journey, the sun’s heat and the enzymes in the lining of the stomach pouch changed the milk into curds and cheese whey. Traditionally, cheese was made as a way of preserving the nutrients of milk. In a simple definition, cheese is the fresh or ripened product obtained after coagulation and whey separation of milk, cream or partly skimmed milk, buttermilk or a mixture of these products. It is essentially the product of selective concentration of milk. Thousands of varieties of cheeses have evolved that are characteristic of various regions of the world. Cheese making is the most traditional method of preserving a food as valuable as milk. More than 1000 varieties of cheese are produced worldwide. On the action of chymosin (rennet), an aspartic acid protease, causes the clotting of milk, a process which involves cleavage of a single peptide bond in k-casein between Phe 105 and Met 106, releasing the acidic C-terminal peptide. This is followed by Ca induced aggregation of the modified micelles to form a ________________________________________________________________________________ Lydia ETCHEBEST & Frédéric RIVAL 15 Enzymes technology in food processing Dr B. Lee gel (Uhlig, 1998). Proteolytic enzymes such as trypsin also cause clotting, but then further degrade casein. If this occurs in cheesemaking it leads to undesirable flavors. Proteins do not contribute significantly to the flavor of foods; it is the peptides and amino acids that often account for the sweet, sour, bitter, and salty flavors. Taste and texture of cheese are altered by the enzymatic conversions taking place during the ripening process. These include the native milk enzymes such as oxidases, phosphatases, peroxidases, catalases, amylases, and lipases, and the exogenous enzymes from rennet that are added to cheese and influence its ripening process. Proteolytic and lipolytic degradative reactions are significantly involved; these cause the formation of even-numbered fatty acids (From C to C odd-numbered methyl ketones (from C to C and a-ketoacids (Uhlig, 1998). All these degradative products play a role in the ripening process. Using genetic engineering, the gene for the enzyme, named chymosin, can be cut out of a calf cell DNA and inserted into the DNA of a yeast or bacterial cell. The microorganism then can make an exact copy of the calf enzyme. Yeast replicate and grow rapidly, so yeast is often used to duplicate the enzyme. Cheese making can be divided into two stages, clotting and aging. In the clotting of milk by rennets two simultaneous processes must be distinguished, namely: - The enzymatic conversion of k-casein into para-k-casein, which is characterized by the rate of proteolysis. The coagulation of the micelles of paracasein produced by the rennet. The latter process is characterized by a flocculation rate constant. To make cheese, bacteria and enzymes are used. The enzyme splits kappa-casein, a major milk protein, causing the milk to clot. The resulting curds and whey are separated. The curd is used to make cheese. For proper aging, the action of specific microbes is needed. Different strains of microbes are used for each type of cheese,. Cheesemaking is really the removal of water from milk. (Milk is 87% water and 13% solids.) This is done by coagulating the protein in the milk. Coagulation changes the chemical makeup of protein so it is no longer water soluble (does not dissolve in water). Heat or acid at the proper temperature coagulates protein. Milk enzymes and microbial enzymes degrade fat, protein, and carbohydrates to different degrees, producing a complex mixture of compounds we call cheese. Bacteria and fungi are living organisms, and as they grow the chemical changes they make as they metabolize milk result in cheeses of different textures and tastes. Scientists discovered that the enzyme rennin (produced in calf stomach lining cells) would coagulate the protein (casein) in milk, forming curds and whey. Because the enzyme reacts with a protein, the enzyme is called a protease. Now, through biotechnology, the gene from the calf stomach cell which makes the cell produce the enzyme rennin is removed and inserted into a bacteria or yeast cell. This causes the organism to produce the enzyme. In cheese, coagulation is done through several steps: - Bacteria convert lactose to lactic acid (same as in yogurt). Allowing the bacteria to work for 30 minutes creates an acid environment. If enough time passed, this acid would eventually coagulate the protein. - Instead of waiting for this, cheese makers use an enzyme called rennet ________________________________________________________________________________ Lydia ETCHEBEST & Frédéric RIVAL 16 Enzymes technology in food processing Dr B. Lee Rennet is an enzyme extracted from the lining of a calf’s stomach, vegetarian rennet is also available this is made from a plant known as ladies bedstraw. Vegetative rennets are made also from fungal origin with Mucor mehei being the most commonly used Its features are: - Rennet is found naturally in the fourth stomach of cows. - Rennet can be man-made to copy the natural rennet works by altering protein so that it is no longer soluble in water (coagulates it). - Rennet works best at 90° and in an acid environment. Therefore, bacteria is added first, to create that acid environment, and then rennet is added. Flocculation rate constants of paracasein micelles vary during the process of renneting. It is therefore added to the milk to turn it from the liquid state to a solid state. The active enzyme in calf rennet is known as Chymosin. In the beginning, when only relatively small amounts of kcasein have been split by the enzyme, they are small and give rise to the formation of loose flocs or gels. In the end, when all k has been converted, they clot extremely rapidly, because the whole micellar surface has become coagulable. At this stage more compact flocs will be formed and syneresis is likely to start. The following is a brief account of recent studies of the renneting reaction, with some emphasis upon the factors which are important in determining the texture of the curd and the clotting-to-proteolysis ratio of different rennets. In the clotting of milk two processes must be distinguished: casein micelles rennet paracasein micelles and paracasein micelles aggregate into curd. The distinction may sometimes be difficult because both processes occur simultaneously or overlap to a considerable extent. Accordingly, when considering the rate of milk clotting, one should also distinguish between the rate at which the paracasein is produced and the rate by which it aggregates into the curd. Casein : Alpha and beta casein: 85% k casein: 12% which is located on the outside of the micelle to protect the rest of the casein from coagulation ________________________________________________________________________________ Lydia ETCHEBEST & Frédéric RIVAL 17 Enzymes technology in food processing Dr B. Lee The milk-clotting process is triggered by the highly specific splitting of the k-casein molecules by the rennet into two parts, differing widely in solubility. The smaller of these is the k-casein macropeptide, which, as a consequence of its excellent solubility, readily dissolves in the milk serum. The other part, the para-k-casein, remains with the micelle and gives it a highly waterrepellent surface. As a consequence, the micelles start to coagulate. Finally it should be mentioned that an average casein micelle is stabilized by thousands and thousands of k-casein molecules. The specific splitting of the k-casein by the rennet is in itself sufficient to render the micelles coagulable, and the Swiss worker Nitschmann demonstrated more than 25 years ago that practically any kind of proteolytic enzyme might do that job. Nevertheless only a few of these are fit to produce a good cheese, as we all know! This is because for clotting we like the enzyme to stop its activity right after the splitting of that particular bond between residues 105 and 106 in kcasein. But most proteolytic enzymes are not so specific as that and continue also to degrade the rest of the casein, which may severely affect the texture and flavor of your cheese. The quality of different rennet preparations is therefore sometimes characterized by the clotting-to-proteolysis ratio, which, obviously, should be high for a good rennet. However, hard cheeses cannot be made without rennet. Hard cheeses claiming to be rennet free are more correctly 'animal rennet' free. These cheeses are made from microbially produced chymosin, which is classed as fermented or vegetarian rennet. Another enzyme is lipases which are normally present in raw milk and are inactivated during pasteurization. The addition of kid goat lipases are common to ensure proper flavour development through fat hydrolysis. Lipase is an enzyme (protein material) which has the function of breaking down the fats of the milk or cheese. Cheeses which use lipase during their manufacture are Fetta, Romano, Pecorino, Parmesan, and many others. Lipolysis is important in ripening, and the extent to which it occurs depends on the particular cheese. In traditional cheese manufacture the endogenous lipase present in unpasteurized milk was the source. With pasteurized milk in which the endogenous lipase is inactivated, a source of lipase is present in the rennet paste. Exogenous lipase is added for cheese varieties such as Mozzarella, Parmesan, Samso and Romi when an enhancement or acceleration of the lipolytic flavour is required Another two important enzymes used in dairy industry is the 13-galactosidase and lactase. The major applications for -galactosidase and lactase are found in: - liquid milk and milk powder, to improve the product for lactose-intolerant individuals, or to increase the sweetness of milk-based drinks; - concentrated milk products, to prevent crystallization of sugars; - fermented milk products, to increase the fermentation rate; - whey as an animal-feed additive, to increase feed intake; - whey as food ingredient, to increase sweetness and prevent crystallization (applied in ice cream, confectionery and bakery products). ________________________________________________________________________________ Lydia ETCHEBEST & Frédéric RIVAL 18 Enzymes technology in food processing Dr B. Lee 3.2 Meat tenderization The most important properties that the meat consumer demands are juiciness, good chewability without loss of firm texture, color, and taste. Native meat enzymes, the cathepsins, play a special role in tenderizing meat by controlled aging. Many ethnic populations have long used meat tenderization as a common kitchen practice. Wrapping meat in papain leaves or dipping or immersing in papaya or pineapple juice is a standard practice in tropical countries. Industrial meat tenderizing enzymes have been used since about 1940. On a commercial scale, plant proteases such as papain (from papaya) and bromelain (from pineapple) are used. These proteases are capable of digesting connective tissue and muscle protein. In addition to PH, temperature and the enzyme quantity used, the delivery of the proteolytic enzyme into the tissues and its distribution therein is important (Uhlig, 1998)). If preparations are sprinkled on the surface of the meat, the interior of the meat remains tough. Repeated injection under pressure into meat is another possibility. Intravenous injection a few minutes before slaughtering has also been studied. Papaya juice has been used for centuries in South America to tenderize meat. It involved injecting papain, an enzyme made from papaya, directly into the bloodstream of living animals. The enzyme papain breaks down tough meat fibers. The white powder sold as "Meat Tenderizer" is composed mainly of an enzyme extract from the papaya, called papain, usually with added salt, sugar and anticaking agents. Although meat texture, especially tenderness, is highly variable, the skeletal muscles of different vertebrates have similar structural and chemical properties. All of them are composed of approximately 75% water, 20% protein, various amounts of lipids and carbohydrates, and a small amount of soluble organic compounds. However, muscles differ greatly in their rates of physiological reaction, reflecting the proportion of each fiber type. There are four major functionally specialized groups of fibers: - slow-twitch-oxidative or type I - fast-twitch-oxidative-glycolytic or type IIA - fast-twitch-glycolytic or type IIB - intermediate or type IIC These fibers contain qualitatively different contractile proteins and both proteinase and inhibitor systems as well as different amounts of myoglobin, oxidative and glycolytic enzymes. The composition of these proteins is well designed for their functional roles. Because of this variability in fiber type composition, one could expect a heterogeneous pattern of post-mortem behavior between or within muscles, and between adjacent cells. Toughness, its opposite, has been considered to be due to the background toughness resulting from the changes in connective tissue, and due to the myofibrillar toughness resulting from the contractile apparatus. The myofibrillar toughness is considered to be affected by the development of rigor-mortis and tenderization caused by enzymatic break-down of the contractile proteins. The enzymes involved in the tenderization process are assumed to be indigenous muscle proteinases which are active at the post-mortem pH of muscles. In fact, Rigor mortis is the first step in the conversion of muscle into meat. Due to the accumulation of lactic acid and protons formed during glycolysis, the pH of meat decreases with ________________________________________________________________________________ Lydia ETCHEBEST & Frédéric RIVAL 19 Enzymes technology in food processing Dr B. Lee the duration of post-mortem. The rate of pH decease is highly related to the contractile and metabolic type of muscle and to meat texture. The rate and extent of pH decline depend primarily on: - the levels of glycogen and energy rich phosphate compounds at death - the rate of ATP turn-over - the buffering capacity of muscle tissue And thus variation in meat tenderness either occurs at slaughter or develops during the postmortem storage, or occurs as a combination of both. The development of toughness explains with the sarcomere shortening and/or the state of actin/myosin interaction hypothesis Meat tenderization during storage of carcasses at refrigerated temperatures has been studied in many laboratories. Current evidence suggests that proteolysis of key myofibril1ar and associated proteins is the cause of meat tenderization. Dissolution or breaks in the I-band at the position of the Z-line have been observed in conditioned meat from different species. Also, many biochemical changes during post-mortem tenderization have generally been assumed to arise from the release of endogenous muscle proteases which are active at the post-mortem pH of muscles. Many studies have been conducted to clarify the mechanism of muscle tenderization. It has been variously postulated that muscle tenderization results from the disappearance of Z-disks, dissociation of actomyosin complex, destruction of connectin or denaturation of collagen. Muscle Proteinase and Meat Tenderization The mechanisms involved in the meat tenderizing process are considered to be enzymatic and physi-cochemical reactions. In living muscles, intracellular protein degradation is controlled, at least partly, by a number of different endogenous proteolytic systems. As most of the post-mortem changes occurring in the process of meat tenderization are considered to be the result of proteolysis, proteinases located inside muscle cells or cytosol can be potential contributors to meat tenderness. Proteasome or macropain which are very interesting are m- and m-calpains, and lysosomal proteinases, namely, cathepsins D, B, H and L. There are four phenomena which reveal the involvement of calpain in post-mortem muscle tenderness: - The ultrastructural degradation of post-mortem myofibrils is quite similar to that of myofibrils treated with calpain. - Post-mortem myofibrillar proteins, untreated or treated with calpain have similar electrophoretical degradation patterns. - The Z-disk, where the calpain localized, is extremely susceptible to calpain-catalyzed hydrolysis. - The higher the level of calpains in muscle, the faster the rate of post-mortem tenderization. However, the concentration of calcium and the existence of the specific endogenous inhibitor for calpain, calpastatin were ignored. As the endogenous protein inhibitors may constitute a powerful regulatory system for muscle proteinases, there is an interest in their identification and characterization. ________________________________________________________________________________ Lydia ETCHEBEST & Frédéric RIVAL 20 Enzymes technology in food processing Dr B. Lee There are many proteases but some are considered a possible candidate for involvement in postmortem tenderization. Some criteria must be checked: - The protease must be endogenous to skeletal muscle cells. - The protease must have the ability to reproduce post-mortem changes in myofibrils in vitro. - The protease must have access to myofibrils in tissue. The protease: calpain has all of the above requirements. If a protease has none of these characteristics, it does not belong to the post-mortem tenderization process. The underlying mechanism of meat tenderization during refrigerated storage of meat occurs thanks to the proteolysis of key myofibrillar proteins by calpains. The factors of tenderness are the resistance of myofibrillar proteins to calpains and the regulation and stability of calpains in muscles. Until now, no direct relationship between muscle proteinase content and the rate of tenderization has been found, but a positive relationship was found in the activity ratio of proteinase to inhibitor. The calpains are considered to be responsible for meat tenderization. Differing from the calpains, which are considered to specifically attack certain proteins of the Z-line,; cathepsins preferentially attack myosin and actin. Furthermore, they can attack contractile proteins at different points. Cathepsin B can rapidly degrade myosin heavy chains while cathepsin L degrades the troponins T and I, and C-protein rapidly, and degrades myosin slowly. Although calpains have an optimum pH range for activity near neutrality, cathepsins, especially cathepsin B and L, have pH optima which are more closely associated with the pH range (5.5~6.5) found in many post-mortem skeletal muscles. Proteases might still be located in lysosomes and have no access to myofibrils or cytosol. However, the fall in pH during post-mortem glycolysis weakens the walls of organelles such as lysosomes, which consequently causes the release of lysosomal proteinases, such as cathepsins B, H, and L, that have pH optima at around 5.5~6.5. Furthermore, when aged muscle is extended, fractures mainly appear close to the Z-lines and, though less frequently, at the junctions of Abands and I-bands. Fragilization of these regions in stored meat can perhaps be ascribed to the action of lysosomal proteinases. A number of in vitro studies have clearly demonstrated the high susceptibility to proteolysis of numerous myofibrillar proteins by calpains and lysosomal proteinases. The general findings summarized above lead to the conclusion that almost all the modifications so far indentified at the myofibrillar structure level in aged meat can be explained by the proteolytic action of muscle proteinases. The post-mortem tenderization of meats needs the synergistic contribution of calpains and lysosomal proteinases. Although most scientists recognize that the weakening of the Z-disk by Cadependent proteinases contributes to meat tenderness, the fragilization of myofibrils at the junctions of A-bands and I-bands or in myosin domains due to lysosomal proteinases, which can lead to total transversal disruption of the structure, might also be an important factor. Even though ________________________________________________________________________________ Lydia ETCHEBEST & Frédéric RIVAL 21 Enzymes technology in food processing Dr B. Lee myofibril rupturing is not complete during the tenderization process, it seems likely that the weakening of the structures involved is sufficient to cause a significant decrease in the mechanical resistance of myofibrils. In conclusion, meat tenderization is a very complex multifactorial process which is con-trolled by a number of various endogenous proteinases and, as yet, poorly understood biological parameters. More detailed investigation is indeed needed to clarify which of the parameters so far discussed, including shortening, pH-temperature-induced release of cytosol calpains and/or membrane-bound lysosomal enzymes, electrical stimulation (ES) induced release of proteases, pH decline, filament disrupture etc., has the largest impact on the tenderness of meats at temperatures which cause minimal cold shortening. Sites of Proteolytic Enzyme Action Suring Ageing: - Calpains: Troponin T (pH > 6), Z-line (desmin), Connectin (gap filaments), M-line proteins, Tropomyosin - Lysosomal enzymes (including cathepsins B, D, H and L), Troponin T, Troponin I, Cproteins, Myosin Heavy Chain, Myosin Light Chains, Actin, Tripomyosin,. Nebulin, Titin, a-Actinin, Collagen (Cross-links of non-helical telopeptides), Mucopolysaccharides Model of the involovement of proteinases in meat tenderization ________________________________________________________________________________ Lydia ETCHEBEST & Frédéric RIVAL 22 Enzymes technology in food processing Dr B. Lee 3.3 Breadmaking Bread has always had a special meaning in human nutrition. Europeans obtain approximately half of their required carbohydrates and about one-third of their protein from bread; in addition, it contains other vital components such as vitamins, minerals, and trace elements. Wheat endogenous enzyme systems and yeast enzymes play a major role in the baking process. Wheat, and consequently wheat flour, contains a wide range of enzyme activities; these different endogenous activities can vary greatly depending for example, on growing or harvesting and storage conditions. A well-known example is wheat a-amylase. Too high activities (e.g. as a result of sprouting) render a wheat unfit for breadmaking. Conversely, a too low activity results in a suboptimal product. The desire to correct for this and to optimize wheat endogenous enzyme levels with enzymes from other sources constitutes the start and general rationale for the use of enzymes in the baking industry. The need for adding enzyme preparations is due to the fact that flour, especially high-quality flour, contains insufficient enzymes for particular applications. Malt is one source of enzymes widely used in the baking industry. It contains a whole range of enzymes including the enzyme diastase, which can be used to compensate for too low endogenous a-amylase levels. Diastase was the first enzyme purified. Payen and Persoz isolated this enzyme in 1833 from malt and demonstrated its ability to convert starch into sugars. The earliest research to use and produce enzymes industrially was performed by Otto Roehm in 1906. Roehm used pancreatic tissue from offals to produce trypsin, which was used in tanning of hides. Nowadays, plants, offal and especially micro-organisms are the sources of enzymes. In order to produce consistent products for the consumer and to make operations more efficient, enzymes are used as supplements in the bread making process. These include xylanase, α-amylase, protease, glucose oxidase and lipase. These are blended into the dry flour and (like the wheat enzymes) are activated when the water is added to make the dough. These supplements enable better handling of dough, and control of characteristics in the finished bread such as taste, loaf volume, crumb texture, and anti-staling properties. Gradually these are replacing other chemical flour improvers. A feature of baking enzymes is that they are usually required to be thermo-labile. This means they are denatured to inactive protein during the baking process Amylases The main application of amylases in baking is in bread-making. Literature data show that amylases can be used to improve or control dough-handling properties and product quality (i.e. volume, color, shelf life). Fungal amylases are widely used, but bacterial amylases seem to be favored for anti-firming. The main drawback of bacterial amylases is their high thermo stability. This problem may be solved since, in recent literature, a bacterial amylase with a thermostability corresponding to that of malt amylase has been reported. In addition, it is reported that the dosage of bacterial amylase is very critical with regard to dough softening. It is standard procedure to make minor adjustments to the amylase levels of milled baking flours by the addition of small levels of fungal alpha amylase. Recent studies in many centres have shown that enzymes other than simple alpha amylase can contribute to the performance of the flour. It is also noted that application of nonamylase enzymes in addition to regular levels of amylase can make substantial improvements to flour behaviour. ________________________________________________________________________________ Lydia ETCHEBEST & Frédéric RIVAL 23 Enzymes technology in food processing Dr B. Lee The application of glucoamylases clearly deserves further study. Literature on glucoamylases is scarce but positive. At present, glucoamylases seem to be used predominantly for brewing andlor starch liquefaction but their use in bread-making looks promising. Also amylase is required to hydrolyse starch to provide glucose for yeast fermentation: it is the latter which produces the CO2 necessary for expansion of the bread dough i.e. for rising to occur. Thus the endogenous glycolytic enzymes of yeast are required for oxidation of glucose formed from starch. The action of amylase to alter the structure of starch also improves the gas retention by the dough. The amount of endogenous amylase activity in flour is variable and depends upon the source and harvest conditions of the grain used to prepare the flour, so that addition of exogenous amylase is frequently made, either during the milling of the flour or in the initial mixing of components to prepare the dough. Fungal amylase addition leads to a dough which has better handling properties and which has a fine crumb structure. The fungal enzyme is inactivated after 10 min at 75ºC. Bacterial amylases may be cheaper than those from fungi but care has to be taken if a bacterial amylase is added which is relatively heat stable as excess activity of such an enzyme produces a sticky crumb in the loaf. The activity of amylase is also required to provide the reducing sugar, i.e. glucose, to provide the colour of the final loaf of bread. A high activity of endogenous amylase the excessive activity of this enzyme degrading the starch can reduce water binding and swelling of starch producing a loaf with a poor volume. The activity of the amylase may also contribute to the dark colour of the crumb and surface of the loaf and the sweet taste. Proteinases Proteases can be used in baking for two completely different purposes. The first, destruction of gluten protein cohesiveness, seems straightforward. Thus papain or the neutral protease from B.subtilis can be applied without problems in the manufacture of wafers, cakes and crackers. When some cohesiveness is still required, as in the case with biscuits and cookies, more care is needed. In bread making, where a selective and controlled modification is needed, problems arise in selecting the right protease. Current assays for protease activity lack relevance to the actual applications of proteases in the manufacture of bakery products. A simple question that has not yet been answered is whether it is the breakdown of structure through which a protease acts, or the breakdown products themselves that causes the observed effects. Detailed studies of the action of enzyme-modified gluten could help to answer this question and obtain the insight needed. The action of proteases can improve elasticity and handling properties of doughs and give bread with a good volume. Most grains used for flour production have low endogenous protease activity as the protease action normally increases once germination of the grain occurs. Addition of a fungal protease, prior to dough mixing, may be beneficial for bread manufacture as this can soften gluten specifically and has a limited activity. Over-activity of added bacterial proteases or the plant protease, papain can lead to decomposition of bread structure through excessive protein hydrolysis. These proteases may be added to high protein flour used for biscuit manufacture where a dough which is easy to roll out and does not rise much is required. The choice of protease is limited by the action of endogenous protease inhibitors in flour and the pH of the dough. The latter is about pH 5.5-6.0 for wheat dough but may be a lower pH (4.2) for rye dough. The latter may be prepared as sour dough. Acidification occurs through the activity of lactic acid bacteria. The rye dough is leavened by its endogenous microorganisms or by addition of a starter culture. ________________________________________________________________________________ Lydia ETCHEBEST & Frédéric RIVAL 24 Enzymes technology in food processing Dr B. Lee Pentosanases In contrast to amylases and proteases, little literature is available on the use of pentosanases in the baking industry. Clearly, this is a new area of application of enzymes, since the increase in the market for whole-wheat breads and high fibre breads provides a potential application for pentosanases. However, several factors limit the skilful and successful application of pentosanases. One of these factors is the complexity of the hemicellulolytic enzyme systems produced. A single micro-organism may produce a series of pentosanases differing in specificity. Yet, it has not been demonstrated clearly whether there are differences in applicability between, for example, Aspergillus pentosanases and Trichoderma pentosanases. It is also not known whether endopentosanases are preferred over exo-pentosanases or whether a certain ratio between the two is required for optimal enzyme action. Many different aspects remain to be studied and many new applications need to be carefully explored. In the rye dough the pentosans play a vital role in dough formation. Rye flour prepared from rye obtained in a dry harvest has few enzymes which give a tough dough and low volume bread: addition of pentosanase can produce a less tough dough, improve volume, give a softer crumb and better storage properties. Careful balancing of proportions of endogenous and added amylase, protease, pentosanase and lipoxygenase activity can improve volume, crumb structure and shelf life. Thus enzymes are important in the formation, the appearance, the flavour and quality of every loaf of bread manufactured. The interactions between enzymes on the various components of bread dough is a very complex subject and the wrong enzyme cocktail or even the wrong application rate can result in detrimental effects on either the dough or the finished bread. For example too much enzyme usually results in a loss of structure, resulting in bread that does not rise properly or that will cause problems in bakery machinery because it is too sticky. 3.4 Enzymes That Aid Beverages In beverages, as in other food products, enzymes may occur naturally or their presence may be due to intentional formulation. Enzymes perform many functions in beverages. They can help increase yields, form nutrients for the fermentation process, facilitate processing, and affect the color, flavor and clarity of the finished product. 3.4.1 Explaining enzyme action Enzymes are biological catalysts based mainly on protein. Because they act as catalysts, the molecules remain unchanged at the completion of the reaction. The exact mechanism by which they perform their function is unknown, although science has developed a number of theories. And although the enzymes can theoretically be recovered, in most food and beverage processes they are merely deactivated by heat upon the completion of the desired reaction because of the expense that would be incurred if the processor tried to recover them. ________________________________________________________________________________ Lydia ETCHEBEST & Frédéric RIVAL 25 Enzymes technology in food processing Dr B. Lee Enzymes are typically named for the reactions they catalyze. They fall into six major categories: oxidoreductases, hydrolases, lysases, transferases, ligases and isomerases. Of these types, hydrolases play the most important role in the beverage industry. Each category contains several types of enzymes; esterases, glycosidases and peptidases are all hydrolases, for example. These may be known by a common name and by scientific nomenclature that describes the reaction they catalyze. Beta-1-4-glucan 4-glucanhydrolase is generally referred to as cellulase. Many industrial enzyme preparations contain a mixture of different types of enzymes. The combination of main and side activities gives a particular preparation its unique functionality. Enzyme isolation and purification increase the cost. Most enzymes catalyze highly specific reactions. For example, alpha-amylase (alpha-1-4glucan glucanhydrolase) attacks the alpha bonds between the glucose portions of starch, resulting in the formation of glucose. Not all enzymes show the same degree of specificity, however. Some proteases, such as papain, hydrolyze random peptide bonds in proteins. A number of factors affect enzyme activity. These include temperature, pH, concentration, contact time with the substrate, trace metals, salt and salt ions, and oxidizing agents. Enzymes are assayed in terms of their activity, expressed as "units." This refers to the amount of substrate catalyzed. Throughout the industry, a wide range of different techniques are used, so anyone attempting to make a direct comparison must know the exact method used. As mentioned, enzymes in beverages can occur naturally in the ingredients used to formulate the beverage. Most fruits contain low levels of pectinase, and the malting process produces significant levels of amylase. In other cases, a product designer can add an enzyme preparation to achieve a specific goal or to supplement or standardize naturally occurring enzymes. 3.4.2 The brew crew Enzymes play a crucial role in the production of beer and other types of malted liquor, such as whiskey. In these products, enzymes provide three major functions: the formation of sugars to be used during fermentation; viscosity control; and, in beer, "chill-proofing." The starting material for beer and whiskey is malted grain. In beer, the grain used is generally barley, although other grains such as corn can augment the barley. Germinating the barley produces a mixture of enzymes, primarily alpha- and beta-amylases. These break down the starch into fermentable sugars such as glucose, maltose and maltotriose. Modern brewers often supplement these naturally occurring enzymes, especially when grains other than barley are used. The additional enzymes can help make up for the lack of amylases in the other grains and increase the level of fermentable sugars. For example, adding amyloglucosidase, which is not found in either the barley or the fermenting yeast, can break down certain dextrins that remain unaffected by the alpha- and beta-amylases. Added enzymes can also ________________________________________________________________________________ Lydia ETCHEBEST & Frédéric RIVAL 26 Enzymes technology in food processing Dr B. Lee standardize the enzymatic conversion of starch, reducing variation in the process and the finished product. The next process where enzymes play a role is the breakdown of the beta-glucans and pentosans. Left intact, these compounds absorb fairly high levels of water, increasing the viscosity and adversely influencing processing. The beta-glucans in particular tend to produce excessive gumminess. Enzymes also serve an important function for those looking for a sparkling, clear beer, especially those designed to be served at lower temperatures. The beta-glucans can cause some of what the industry refers to as haze, but most of this problem comes from protein precipitation. "Chill-haze" is caused by the hydrogen bonding of protein and tannin, and it is reversible. These two compounds can also form a complex that does not redissolve when the temperature rises. To solve this problem, papain is added after brewing. Other processes such as filtration and protein flocculation have been used to remove protein. 3.4.3 From the vine Wine-making generally relies on the naturally occurring enzymes present in the grape or formed as a product of fermentation. These influence the color and flavor of the finished product. Still, added enzymes could help in several areas. Added pectinase can aid in pressing and clarification. It would be particularly helpful during any type of elevated heating during the mash process, since higher temperatures at this stage mean increased levels of pectin in the juice. This excess pectin not only affects the finished product, but also influences the viscosity during processing. Pectinase can accelerate the release of pigments from the grape skin, as well. Sweet wines, such as sauternes, depend on grapes treated with Botrytis cincerea (a mold that causes the grapes to dehydrate, thus concentrating the sugar). This organism produces a glucan polymer that can interfere with filtering. Therefore, it has become common to use a specific glucanase to break down this molecule prior to filtration. 3.4.4 Serving tea and cocoa Green tea leaves must undergo a fermentation step to create the colors, flavors and astringency associated with black tea. This fermentation is actually an enzymatic degradation of various compounds. Naturally occurring polyphenoloxidase and peroxidase cause most of the desired chemical changes, but other enzymatic reactions probably help develop the flavor by creating ________________________________________________________________________________ Lydia ETCHEBEST & Frédéric RIVAL 27 Enzymes technology in food processing Dr B. Lee aldehydes and terpenes. In addition, catalase affects the level of peroxide available for the peroxidase to act on. This enzymatic activity creates the need for another traditional step: "firing". When the reaction has proceeded to the required level, the leaves are heated to approximately 190 degrees F to inactivate the enzymes. As always, science has found ways to improve on nature. The caffeine and polyphenols in tea complex to form insoluble complexes in cold conditions, creating the "tea cream" seen in iced tea. Enzymatically modifying this complex with tannase increases the cold water solubility of tea solids and produces a clear tea beverage. Those who want chocolate milk or hot cocoa also rely on enzymes. In addition to a microbial fermentation when processing the cocoa bean, both native enzymes and enzymes produced by the yeast and bacteria provide several functions. They help break down the membrane between the bean and pod. They may help generate reducing sugars inside the bean that give the roasted product its characteristic flavor. Proteases create flavor precursors, and polyphenoloxidase helps develop the color. 3.4.5 A very juicy story The juice industry relies heavily on enzymes to extract juice from fruits and prepare a finished product. For non-citrus juices, such as apple, grape and berry, processors add enzymes at the beginning of the mash stage to help extract juice. The cell walls of fruits consist of cellulose, hemicelluloses, pectin and proteins. Pectin is the major structural polysaccharide. Adding the appropriate enzymes to break down these structures makes it possible to extract a larger amount of juice, to facilitate pressing, and to produce a clear juice. Adding cellulase, hemicellulase and pectinase during or prior to pressing helps release the juice contained within the cell walls, increasing yield. It can also save wear and tear on pressing equipment and reduce energy requirements, especially when juice is extracted from a fruit with a very firm structure, such as cranberries. Some enzymatic processes allow the entire fruit to be liquefied. The most prevalent enzymes used in juice processing are pectinases: primarily pectin methyl esterase (PME) and polygalacturonase (PG), although some pectinases may be used. The PME first removes the methyl groups from the pectin molecule. The activity required varies from fruit to fruit because different fruits have different levels of methoxylation. Once this step is completed, the PG can break down the main pectin chain by catalyzing hydrolysis. Pectin lyase can also split the pectin polymer. ________________________________________________________________________________ Lydia ETCHEBEST & Frédéric RIVAL 28 Enzymes technology in food processing Dr B. Lee These enzymatic reactions to the pectin molecule not only increase yield, they clarify the juice. The cloud consists of small particles made up of pectin/protein complexes suspended in the juice. These create a haze. Adding enzymes that help break down the pectin changes two things. First, the viscosity of the juice decreases. Second, the enzymes cause ions to form, resulting in electrostatic aggregation which increases the size of the particles. The combination of decreased viscosity and increased particle size causes a floc that settles out, and the clarified juice can be removed. Amylase and amyloglucosidase may also be required to break down any starch present in the fruit. Starch becomes a problem when using immature fruit, such as green apples. If not broken down, the starch can form large polymers in the juice that create a haze. For some juices, particularly orange juice and fruit nectars, a cloud is a desirable attribute. In these cases, any naturally occurring or added pectolytic enzymes must be inactivated by heat. However, these juices may need reductions in viscosity. In these products a controlled breakdown of pectin is sometimes done using polygalacturonase or pectin lyase. 3.4.6 Color and flavor concerns for fruit juice Enzymes in fruit juice also affect the color and flavor of the juice. Many of the naturally occurring enzymes help to form esters, aldehydes and alcohols -- all important flavor volatiles in fruit. Enzymes can affect the anthocyanins, the major pigment in berry and grape juices. "The correct level of enzymes helps preserve the color. However, excess dosages can reduce or break down color," warns Ware. Enzymes have been used in the debittering of citrus juice, particularly grapefruit juice. The major bitter compound is a flavonone, naringin. Adding nariginase (a combination of enzymes containing alpha-rhamnosidase and beta-glucosidase) breaks the molecule down to nariginin and prunin, reducing the bitterness. 3.4.7 Dairy doses The other class of beverages that can benefit from the addition of enzymes includes milk and other fluid dairy products. The enzymatic process of interest is the hydrolysis of lactose. ________________________________________________________________________________ Lydia ETCHEBEST & Frédéric RIVAL 29 Enzymes technology in food processing Dr B. Lee For the lactose-intolerant, the lactose in milk can pose serious medical problems. These people cannot produce enough beta-galactosidase in their digestive system to break down this sugar. Lactase, a beta-galactosidase, catalyzes the hydrolysis of the beta-D-galactoside linkage of lactose, converting it into glucose and galactose. Lactase is produced commercially through the controlled fermentation of certain microorganisms, including Kluyveromyces lactis and Aspergillus oryae. Although some oral preparations of this enzyme are on the market, the milk itself can be treated, resulting in a low-lactose or lactose-free product. "To treat milk, the lactase is injected directly into the pasteurized milk. During the storage time, the lactose is hydrolyzed to glucose and galactose. The yeast-derived lactase operates well under neutral pH conditions and at temperatures between 3 to 25 degrees C. 3.5 Starch & sugar Industries Considerable quantities of the sweeteners used throughout the world are derived from starch as opposed to cane or beet sugar. The enzymatic treatment of starch has become much more popular than acid hydrolysis. The treatment of starch with enzymes results in a variety of sweet syrups used throughout the food and beverage industries. Three stages can be identified in starch modification. Firstly, amylases liberate "maltodextrin" by the liquefaction process. Such maltodextrins are not very sweet as they contain dextrins and oligosaccharides. The dextrins and oligosaccharides are further hydrolysed by enzymes such as pullulanase and glucoamylase in a process known as saccharification. Complete saccharification converts all the limit dextrans to glucose, maltose and isomaltose. The resulting syrups are moderately sweet and are frequently modified further. Treatment of glucose/maltose syrups with glucose isomerase converts a large proportion of the glucose to fructose which is sweeter than glucose. This isomerisation process is usually performed with immobilised glucose isomerase and results in syrups with approximately 50 % fructose and 50 % glucose. Such products are known as high fructose syrups and are frequently used as "sugar replacements" in the manufacture of foods and beverages. The high fructose syrup is greater in demand than pure glucose as food and drink sweeteners, because fructose is sweeter than glucose. Therefore, if glucose can be converted into fructose, its commercial value is increased greatly. The starch industry turned to enzymes for use as a processing aid at a very early point in the proliferation of enzyme applications. The entire process is dependent on high quality enzymes for the production of many grades of starch that is the reason why this move away from harsh ________________________________________________________________________________ Lydia ETCHEBEST & Frédéric RIVAL 30 Enzymes technology in food processing Dr B. Lee chemicals has increased the production yields, allowing manufacturers to apply syrups in new profitable applications. The Enzymes, which are used in sweeteners production 1. a-amyalse: hydrolyses a-1,4 bonds in glucose polymers, but only within chains, yielding shorter chains(dextrins). Obtained commercially from bacteria (e.g. Bacillus spp.). 2. B-amylase: hydrolyses a-1, 4 bonds in glucose polymers, breaking off successive maltose units from the (non-reducing) ends of the obtained commercially from barely and malt. 3. Amyloglucosidase: breaks a-1, 4, cleaving glucose units progressively from the (non reducing) ends of the chain but not slowly. Obtained commercially from the fungi Aspergillus spp. And Rhizopus oryaze. 4. Pullulanase: hydrolyses a-1,6 bonds. Obtained commercially from the bacteria Bacillus acidopullulyticus and Klebsiella pneumonia. 5. Glucose isomerase: transforms glucose into its sweeter-tasting isomers fructose. 3.6 Enzymes in the processing of fats and oils Traditionally, the technology available for fat hydrolysis is high-temperature steam splitting. With growing ecological concern, increasing energy costs, and desire to achieve better yields and quality of fatty acids and glycerin, enzymatic inetesterification is used to change the physical properties of mixtures of fats by randomly redistributing fatty acid groups among the triglycerides. The enzymes used for modification of oils and fats are extracellular microbial lipases. They are excreted by micro-organisms into the growth medium to catalyse the degradation of lipids, and can be produced on a large scale by fermentation. Lipases catalyse the hydrolysis of oils and fats to give diglycerides, monoglycerides, glycerol and free fatty acid. The reaction is reversible, and consequently microbial lipases also catalyze the formation of glycerides from glycerol and free fatty acid. In recent research, methods were developed to recycle the enzyme. Since the target product is a fatty acid, which is a low-price commodity, only the immobilization procedures can be used that retain a high degree of activity after immobilization. Lipase, when immobilized by hydrophobic adsorption, is stable during storage in fatty acids; works at an optimum of pH 4 and 42 °C; purity of fatty acids produced is much better than those produced via steam splitting; and enzyme losses in fatty acid or glycerin can be minimized. ________________________________________________________________________________ Lydia ETCHEBEST & Frédéric RIVAL 31 Enzymes technology in food processing Dr B. Lee 3.7 Other food enzymes from GMOs It has been estimated by some industrial observers that 50% of all industrial enzymes (including food and detergent enzymes) are already being manufactured using GMOs, i.e. the recombinant DNA biotechnolgy. For example, most lipases used in many cold-wash detergents are produced by GMOs, and phytase, a plant derived enzyme that has been engineered into Aspergillus niger, is reportedly used in 50% of all pig feed in The Netherlands (Roller and Goodenough, 1999). The first example of a processing enzyme produced by rDNA biotechnology for use in food was chymosin. The chymosin example established the basis for production of a variety of safe and functional rDNA biotechnology-derived food-grade enzymes. Their accepted use in foods is based on the following facts: enzymes produced by rDNA biotechnology are identical to their natural counterparts; enzyme preparations are free of any deleterious substances that could be introduced during the bioprocessing and purification steps; and viable rDNA biotechnology-derived microorganisms are not present in the final preparation. Some examples of food enzymes produced commercially (or very soon to be produced) with the aid of GMOs are shown in Table 2. The development of enzyme production from GMOs has been driven by three major forces: the need by the enzyme supplier to improve production efficiency (i.e. enzyme yields from fermentation) and to maintain profit margins in the face of declining prices; the need to improve enzymes performance in existing applications; and the demand for enzymes suitable for novel applications at an economic price. Table 2 Commercial and near-market rDNA biotechnology-derived food enzymes (Compiled from Roller and Goodenough 1999). ________________________________________________________________________________ Lydia ETCHEBEST & Frédéric RIVAL 32 Enzymes technology in food processing Dr B. Lee 4. Legal and safety implications Regulations of enzymes used in food in the European Union (EU) Assessment Nancy W. ZemanTriangle Biotechnology ConsultingChapel Hill, NC USA 4.1. Global considerations: Enzymes are natural substances that are produced by all living cells. The application of enzymes to food processing developed from the need for highly specific safe catalysts and has a long history of safe use. Commercial enzymes preparations used in food are derived from plant or animal tissue or from microbial sources. The number of enzymes derived from microbial species has greatly expanded over the last few decades. Traditionally, enzyme manufacturers have continually improved their microorganisms to enhance productivity through selection of production strains containing spontaneous mutations; chemically or physically induced mutations; or additional genetic information introduced by natural processes, such as conjugation or transduction. The advent of genetic engineering and its application to the enzyme industry in the last 20 years has provided another method by which enzyme manufacturers can improve their production strains. The regulation of commercial enzyme preparations and their application to food is generally controlled by national and international legislation and is highly varied throughout the world. Some countries require "approval" before a new product is introduced, others request to be "notified" prior to the sale of an enzyme, while some have no approval/notification requirements at all. Enzymes produced using modern biotechnologies often have additional regulations over those from traditional sources. Many countries, including the European Union, Japan, Australia, and New Zealand, are currently developing or reassessing their regulations for enzymes from genetically modified organisms (GMOs). Pre-market approval of enzyme preparations is often dependent on whether they are classified as processing aids or food additives. There are many different definitions for processing aids and food additives found worldwide; however, from a safety point of view, the classification may be irrelevant, since in either case, the enzyme preparation may end up in the food, thus its safety must be assured. 4.2. Specifications: In 2001, the Joint FAO/WHO Expert Committee on Food Additives (JECFA) completed the update of its general specifications and considerations for enzyme preparations used in food processing. Special consideration was given to updating of specifications in light of recent technological advances. The revised specifications suggest all newly developed enzyme preparations pass through a general safety assessment. Many of the requirements previously outlined for enzyme products from GMOs were appropriate for all preparations, regardless of source, thus the Committee revised the specifications to reflect this. For enzymes from generally modified sources, the general considerations now focus on the final construct intended for use as the source organism, not the method of construction, as the JECFA committee agreed that the ________________________________________________________________________________ Lydia ETCHEBEST & Frédéric RIVAL 33 Enzymes technology in food processing Dr B. Lee potential products from inserted DNA are what should be considered when assessing allergic potential and other safety concerns. The United States regulates enzyme preparations either as secondary direct food additives or as Generally Recognized as Safe (GRAS) substances. Enzymes considered as food additives require pre-market approval from the Food and Drug Administration (FDA) which is obtained through a petitioning process. Enzymes considered as GRAS (either by common use in food prior to 1958 or by scientific procedures) do not require approval from the FDA, but requests for affirmation of GRAS status by the FDA can be made, again through a petitioning process. While this process is often lengthy (one enzyme petition has been on file for more than 25 years) and there is a backlog of petitions at the Agency, these petitioned enzymes may be marketed while under review. 4.3. FDA notifications: In 1997, the FDA issued a proposed rule for GRAS Notifications. This rule established a notification procedure whereby interested parties could notify the Agency of a determination that a particular use of a substance is GRAS. As described in the proposed rule, the Agency evaluates whether the information contained in the notice provides a sufficient basis for a GRAS determination and whether information in the notice or otherwise available to the FDA raises issues that lead the Agency to question whether use of the substance is indeed GRAS. With notifications, the FDA does not affirm the GRAS status for these substances but maintains an inventory of GRAS Notices and the Agency's response to those notices which are posted on the FDA website. The Agency responds in one of three ways: it does not question the basis for the GRAS determination; it concludes that the notice does not provide a sufficient basis for a GRAS determination; or the Agency, at the notifier's request, ceases to evaluate the GRAS notice. The GRAS Notification procedures, though not yet finalized, have in general been well accepted by enzyme manufacturers and enzyme users. Of the approximately 100 GRAS notices that have been submitted to the FDA since 1997, about 20 are for enzyme preparations. There are no specific statutory requirements for GRAS affirmation petitions or notices although guidelines for the chemistry requirements have been published. The burden of proof of safety is on the enzyme manufacturer/distributor. Most U.S. enzyme manufacturers use the decision tree of Pariza and Johnson2 when assessing safety of a new product. In the European Union (EU), the regulation of enzymes is particularly confusing. Most of the enzyme preparations used for food processing in the EU are considered processing aids, i.e., they have no technical function in the final food. As such, their use in food is not currently covered by a community regulation, but this situation is being evaluated. In 2000, a task force (called the SCOOP Task Force) was initiated to examine how enzymes should be classified, regulated, and assessed for safety. The final report from the SCOOP Task Force has not yet issued. Enzymes that do have a technical function in the final food are classified as food additives. At present in the EU, there are only two enzyme preparations that fall into this category. To gain approval for a food additive, one must submit a dossier to the Scientific Committee for Food (SCF). The SCF has issued guidelines for the presentation of data on food enzymes that they consider to be minimum requirements3. ________________________________________________________________________________ Lydia ETCHEBEST & Frédéric RIVAL 34 Enzymes technology in food processing Dr B. Lee Several European countries have legislation covering all food uses for enzymes, regardless of classification. France and Denmark require approval of new enzyme preparations prior to use; in the UK, approval is voluntary, but recommended. 5. Protein Engineering of enzymes A most exciting development over the last few years is the application genetic engineering techniques to enzyme technology. There are a number of properties which may be improved or altered by genetic engineering, including the yield and kinetics of the enzyme, the ease of downstream processing and various safety aspects. Enzymes from dangerous or unapproved micro-organisms and from slow growing or limited plant or animal tissue may be cloned into safe high-production micro-organisms. In the future, enzymes may be redesigned to fit more appropriately into industrial processes; for example, making glucose isomerase less susceptible to inhibition by the Ca present in the starch saccharification processing stream. The amount of enzyme produced by a micro-organism may be increased by increasing the number of gene copies that code for it. This principle has been used to increase the activity of penicillin-Gamidase in Escherichia coli. The cellular DNA from a producing strain is selectively cleaved by the restriction endonuclease md This hydrolyses the DNA at relatively rare sites containing the 5’AAGCTT-3’ base sequence to give identical ‘staggered’ ends. The total DNA is cleaved into about 10000 fragments, only one of which contains the required genetic information. These fragments are individual cloned into a cosmid vector and thereby returned to E. coli. These colonies containing the active gene are identified by their inhibition of a 6-amino-penicillanic acid-sensitive organism. Such colonies are isolated and the penicillin-Gamidase gene transferred on to pBR322 plasmids and recloned back into E. coli. The engineered cells, aided by the plasmid amplification at around 50 copies per cell, produce penicillin-Gamidase constitutively and in considerably higher quantities than the fully induced parental strain. Such increased yields are economically relevant not just for the increased volumetric productivity, but also because of reduced downstream processing costs, the resulting crude enzyme being that much purer. Another extremely promising area of genetic engineering is protein engineering. New enzyme structures may be designed and produced in order to improve on existing enzymes or create new activities. Such factitious enzymes are produced by site-directed mutagenesis. Unfortunately from a practical point of view, much of the research effort in protein engineering has gone into studies concerning the structure and activity of enzymes chosen for their theoretical importance or ease of preparation rather than industrial relevance. This emphasis is likely to change in the future. The preferred pathway for creating new enzymes is the stepwise substitution of only one or two amino acid residues out of the total protein structure. Although a large database of sequencestructure correlations is available, and growing rapidly together with the necessary software, it is presently insufficient to accurately predict three-dimensional changes as a result of such substitutions. The main problem is assessing the long-range effects, including solvent interactions, ________________________________________________________________________________ Lydia ETCHEBEST & Frédéric RIVAL 35 Enzymes technology in food processing Dr B. Lee of the new structure. As the many reported results would attest, the science is at a stage where it can explain the structural consequences of amino acid substitutions after they have been determined but cannot accurately predict them. Protein engineering, therefore, is presently rather a hit or miss process, which may be used with only little realistic likelihood of immediate success. Apparently quite small sequence changes may give rise to large conformational alterations, and even affect the rate-determining step in the enzymic catalysis. However it is reasonable to suppose that, given a sufficiently detailed database plus suitable software, the relative probability of success will increase over the coming years and the products of protein engineering will make a major impact on enzyme technology. Much protein engineering has been directed at subtilisin (from Bacillus amyloliquefaciens), the principal enzyme in the detergent enzyme preparation, Alcalase. This has been aimed at the improvement of its activity in detergents by stabilising it at even higher temperatures, pH and oxidant strength. Most of the attempted improvements have concerned alterations to: 1. the P cleft, which holds the amino acid on the carbonyl side of the targeted peptide bond; 2. the oxyanion hole (principally Asni which stabilises the tetrahedral intermediate; 3. the neighbourhood of the catalytic histidyl residue (His which has a general base role; and 4. the methionine residue (Met which causes subtilisin’s lability to oxidation. An example of the unpredictable nature of protein engineering is given by trypsin, which has an active site closely related to that of subtilisin. Substitution of the negatively charged aspartic acid residue at the bottom of its P cleft (Asp which is used for binding the basic side-chains of lysine or arginine, by positively charged lysine gives the predictable result of abolishing the activity against its normal substrates but unpredictably also gives no activity against substrates where these basic residues are replaced by aspartic acid or glutamic acid. Considerable effort has been spent on engineering more thermophilic enzymes. It has been found that thermophilic enzymes are generally only 20-30 kJ more stable than their mesophilic counterparts. This may be achieved by the addition of just a few extra hydrogen bonds, an internal salt link or extra internal hydrophobic residues, giving a slightly more hydrophobic core. All of these changes are small enough to be achieved by protein engineering. To ensure a more predictable outcome, the secondary structure of the enzyme must be conserved and this generally restricts changes in the exterior surface of the enzyme. It should be recognised that making an enzyme more thermostable reduces its overall flexibility and, hence, it is probable that the factitious enzyme produced will have reduced catalytic efficiency. 6. Future Prospects Enzyme technology is presently going through a phase of maturation and evolution. The maturation is shown by the development of the theory concerning how enzymes function and how this is related to their primary structure through the formation and configuration of their three________________________________________________________________________________ Lydia ETCHEBEST & Frédéric RIVAL 36 Enzymes technology in food processing Dr B. Lee dimension structure. The evolution is shown by the ever-broadening range of enzymic applications. The enzymes are very useful in food technology and the biotechnology produces enzymes, exogenous enzymes which produce homogeneous products. The endogenous enzymes are still used like some French wines but to decrease the costs and to get products without variability because of different qualities of endogenous enzymes, the best solution is to add exogenous enzymes produced from bacteria. Enzymes may be produced from plant or animal origins but it is more expensive and longer. Many organisms currently used in enzyme manufacture produce mixtures of several enzyme activities. For example, some fungal pectinases used in fruit juice processing contain up to 20 different enzyme activities. Although such crude enzyme mixtures are likely to remain on the market because of their low cost and adequate performance in certain applications, it is conceivable that much more sophisticated, tailor made enzyme mixtures prepared from a selected range of single-activity recombinant enzymes will become available in the future, particularly for applications where side-activities are undesirable. There still remains much room for the development of useful processes and materials based on this hard-won understanding. Enzymes will clearly be more widely used in the future and this will be reflected in the number enzymes available on an industrial (and research) scale, the variety of reactions catalyzed and the range of environmental conditions under which they will operate. Established enzymes will be put to new uses and novel enzymes, discovered within their biological niches or produced by design using enzyme engineering, will be used to catalyze hitherto unexploited reactions. This is just the start of the enzyme technology era. ________________________________________________________________________________ Lydia ETCHEBEST & Frédéric RIVAL 37 Enzymes technology in food processing Dr B. Lee References 1. Taylor, A.J., "Enzymes in the Food Industry" in "Enzymes in Food Processing" , (G.A. Tucker and L.F.J. Woods, Eds.), pp. 22-34, Blakie and Son Ltd.,London, (1991). 2. Hui, Y.H. ,"Enzymes as Food Additives" in "Encyclopedia of Food Science and Technology", (Y.H. Hui, Ed.),Vol. 2, pp. 741-743, John Wiley & Sons, Inc., New York, (1992). 3. Van den Tweel, W.J.J., Leak, D., Bielecki, S. and Pedersen, S., "Biocatalyst Production" in "Applied Biocatalysis", J.M.S. Cabral, D. Best & J.Tramper, Eds.), pp. 157-236, Harwood Academic Publishers GmbH, Chur, 1994. 4. Tucker, G.A., "Enzymes. Developing Enzyme Based Processes", International Food Ingredients, 2, 23-26, (1996). 5. Patel, P.D., "Enzymes as Diagnostic Tools" in "Enzymes in Food Processing" (G.A. Tucker and L.F.J. Woods, Eds.), pp.262-283, Blackie and Sons Ltd., London, (1991). 6. Olsen, H.S., "Use of Enzymes in Food Processing, in "Biotechnology", (H.-J. Rehm, G. Reed, A. Puhler and P. Stadler Eds.), 2nd Ed., Vol. 9 (Enzymes, Biomass, Food and Feed), pp. 663-736, VCH Verlagsgesellschaft mbH, Weinheim, (1995). 7. Lea, A.G.H., "Enzymes in The Production of Beverages and Fruit Juices", in "Enzymes in Food Processing", (G.A. Tucker and L.F.J. Woods, Eds.), pp.194-220, Blackie and Son Ltd., London, (1991). 8. Brenna, O. and Bianchi, E., "Immobilized Laccase for Phenolic Removal in Must and Wine", Biotechnol. Lett., 16, 35-40, (1994). 9. Dubourdieu, D. et al., "Mise au Point d'une Mesure Rapide de l'Activité Laccase dans les Moûts et dans les Vins par la Méthode à la Syringaldazine. Application à l'Appreciation de l'État Sanitaire des Vendages", Conn. Vigne Vin, 18(4), 237-252, (1984). 10. Palleschi, G., Rathore, H.S., Mascini, M., "Amperometric Probe for 3-Hydroxybutyrate with Immobilized 3-Hydroxybutyrate Dehydrogenase", Anal. Chim. Acta, 209(1-2), 223230, (1988). 11. Angold, R., Beech, G. and Taggart, J. (1989) Food Biotechnology. Cambridge University Press, Cambridge. 12. Bailey, A.J. and Light, N.D. (1989) Connective Tissue in Meat and Meat Products. Elsevier Applied Science, London, pp. 195-224. 13. Chaplin, M.F. & Bucke, C. 1990. Enzyme Technology. Cambridge University Press. Cambridge, U.K. ________________________________________________________________________________ Lydia ETCHEBEST & Frédéric RIVAL 38 Enzymes technology in food processing Dr B. Lee 14. Cheetham, P.S.J. 1985. The application of enzymes in industry. In Handbook of Biotechnology. Second edition (ed.; Wiseman, A.). 15. Critchley, P. (1987) Enzymatic Modification of Lipids. In Chemical aspects of food enzymes, ed. Andrews, A.T. Royal Society of Chemistry, London, pp 149-155. 16. Denner, H.W.B. (1983). The Legislative Aspects of the Use of Industrial Enzymes in the Manufacture of Food and Food Ingredients. In Industrial Erizymology, eds. Godfrey, T. and Reichelt, J. Nature Press, New York, pp. 111-137. 17. Frost, G.M.; Moss, D.A. 1986. Production of enzymes by fermentation. In Biotechnology. (Ed.;Rehm, H.S.;Reeds, G.)Vol.7a. 18. Fullbrook, P.D. (1983) Practical limits and Prospects. In Industrial Enzymology, eds. Godfrey, T. and Reichelt, J. Nature Press, New York, pp. 4 1-110. 19. Lawrie, R.A. (1985) Meat Science ( edn.). Pergamon Press, Oxford. 20. Open universiteit & Thames Polytechnic 1991 Biotechnological Innovations in Food Processing Butterworth-Heinemann Ltd. Oxford London. 21. Price, N.C. & Stevens, L. 1999. Fundamentals of Enzymology 3 ed. Oxford University Press Inc. New York. 22. Roller, S. and Goodenough, P.W. (1999) Food Enzymes. Genetic Modification in the Food Industry. 23. Uhlig, H. 1998. Industrial Enzymes and Their Applications John Wiley & Sons Inc. New York. 24. Wolnak, B. (1980). Status of he US Enzyme Industry. In Enzymes: The Interface Between Technology and Economics, eds. Danehy, J.P. and Wolnak, B. Marcel Dekker, New York, pp.3-10 . 25. Asai, H. et al., "Method and Indicator for Simultaneously Performing Quality Testing of Animal Milk and Health Testing of the Animals", Eur.Pat. Appl. EP 226,427 (24/jun/87), JP Appl. 85/276,048 (10/Dec.1985). 26. A. Rosevear, J.F. Kennedy and J.M.S. Cabral (Eds), "Immobilised Enzymes and Cells", IOP Publishing Ltd., Bristol, 1987. 27. G.A. Tucker and L.F.J. Woods, (Eds.),"Enzymes in Food Processing", Blakie and Son Ltd.,London, (1991). 28. www.biocatalysts.com 29. www.biocone.com ________________________________________________________________________________ Lydia ETCHEBEST & Frédéric RIVAL 39