Excellence Exemplar for a Fair Test
advertisement

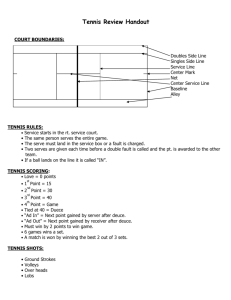
Excellence Exemplar for a Fair Test AS90727 (version 2) Science 3.1 Carry out a practical scientific investigation with guidance How Temperature affects the Bounce of Tennis Balls Initial trialling: INITIAL IDEA: Mr told us about the extended investigation today. I am a keen tennis player and I think that the temperature of the day may affect the bounce of the balls I am using. So I think that I will design an experiment around that idea. PURPOSE: To determine if temperature affects the distance that a tennis ball bounces. HYPOTHESIS: If temperature is increased and all other factors remain the same, then the ball will bounce further. I expect this because in cold conditions air contracts and the ball will act as if it is slightly deflated. INDEPENDENT VARIABLE: The temperature will be measured in degrees centigrade, using a thermometer. My range of temperatures is going to be from 6oC (see notes later)to 26oC. I will vary the temperature by using heaters, and by the time of day that I do an experiment. DEPENDENT VARIABLE: The distance between the balls first and second bounce. To be measured with a tape measure in cm. JUSTIFICATION FOR METHOD: I wanted to use tennis balls also because other hollow balls such as netballs loose air more quickly than tennis balls, which would have introduced an unnecessary error. I trialled two ways of determining the dependent variable. Firstly I dropped the balls from the same height and measured how high the ball bounced. Because I was working on my own I didn’t think that I could judge the height of the bounce very accurately. Then I decided to do the method mentioned next. I designed a ramp so that I could accurately set the ball off from the same height each time. I thought about how to accurately determine where the ball bounced and I used talcum powder on the concrete floor as you could then very accurately record where the ball landed. The ball made a crater when it bounced with a peak in the middle. This meant that I was able to measure from the peak of the first bounce to the peak of the second bounce. I trialled this visually at 7oC and at 20oC and found that I was getting a significant difference between the two temperatures. Method trialled and the reasons for the design explained and justified. EQUIPMENT: Ramp with recorded height, length and angle (the actual dimensions do not matter just record them). Like in picture 3 Tape measure (or long ruler of any kind) Thermometer Talcum powder Tennis balls x3 Heaters Aluminium garage – I chose this because I could keep the garage at a constant temperature as I did the experiment. INITIAL METHOD: 1. Set up your ramp so it is above a smooth and level surface. 2. Sprinkle the floor with talcum powder so the coating is light. 3. Record the temperature. 4. Sweep with a broom or brush so the talcum powder is in lines. 5. Hold the tennis ball at the top of the ramp let it roll down the ramp (do not push it, a block of wood would be a good thing to start it with). 6. Watch it bounce to get a general idea of where it landed on its first and second bounce. Then look at the talcum powder. Look for craters and measure the distance from the peak in the crater caused by the first bounce and the one caused by the second bounce. 7. Record your results. 8. Repeat steps 2-7 5 times using the same tennis ball. 9. Repeat steps 2-8 using a different tennis ball. 10. Repeat steps 2-9 with 5 different temperatures. Note you don’t need a specific temperature just to measure it precisely. 11. Process the data to determine the kinetic energy lost. Initial method written up as part of the trialling. Final Report: How Temperature affects the Bounce of Tennis Balls BACKGROUND: I am a keen tennis player and I practise at all times of the day. I started noticing that the balls I used sometimes seemed to bounce higher in the middle of the day than when I practised early in the morning. I began to wonder if temperature affected the bounce of tennis balls. I decided to make this the topic of my extended practical investigation. I decided to use one brand of balls and test them over a range of temperatures. This made my investigation a fair test. PURPOSE: To determine if temperature affects the distance a ball will bounce. Statement of purpose HYPOTHESIS: If temperature is increased and all other factors remain the same, then tennis balls will bounce further. SCIENCE IDEAS: I expect this because in cold conditions air contracts and the ball will act as if it is slightly deflated. The density, pressure and temperature of a gas are related. When a gas moves against an object, such as the wall of a tennis ball, it pushes on the surface of the object - pressure. Gases can be expanded or compressed; they can expand to fill a new larger volume. The mass doesn't change, but the volume increases, so the density of the gas decreases in the new volume. The structure of a tennis ball has a fabric covering and a hollow inner core of a rubber-like substance. Inside the core is a gas which fills the entire inner core of the ball. A tennis ball bounces because the tennis ball and the gas inside act like a spring. As the tennis ball strikes the court the bottom of the ball is pushed in. The tennis ball material is pliable and deforms (bends). The compressed (pressed in) ball has less volume than the original uncompressed ball. As the ball comes off the court the gas and tennis ball material act like a spring and the ball returns to its original shape. As the temperature increases the molecules of the gas expand and move around more inside the ball. As the molecules move more they strike the inner surface of the ball and apply more pressure to it. Balls with more air pressure in them bounce better because air, when compressed, will uncompress (spring back) with little or no energy loss. A ball that has higher air pressure in it will not squash as much during the collision, and so less energy will be lost. Therefore the ball will bounce further. Explanation of relevant science ideas –could be written up at the beginning of the report or the end as part of the discussion. INDEPENDENT VARIABLE: Temperature to be measured in degrees centigrade using a thermometer. My range of temperatures is to be 6oC, 11 oC, 16 oC , 21 oC and 26oC. Independent variable with valid range DEPENDENT VARIABLE: Distance between the balls first and second bounce, taken from the peak in the centre of the crater in the talcum powder. To be measured with a tape measure in centimetres. Accurate measurement of dependent variable EQUIPMENT: Ramp with recorded height, length and angle Tape measure (or long ruler of any kind) Thermometer Talcum powder 3 tennis balls of the same brand Heaters Suitable cleared space (I used an aluminium garage with a concrete floor) FINAL METHOD: 1. I set up my ramp as in picture 1 so it is above the garage floor, which was a smooth and level concrete surface. 2. I sprinkled the floor with talcum powder so the coating was light and swept it so that there were faint lines across it. 3. I swept the floor with a brush so the talcum powder was in line. 4. I recorded the temperature. 5. I then held the tennis ball at the top of the ramp (62.4 cm) and let it roll down the ramp (I didn’t push it but used a block of wood to start it with). 6. I watched it bounce to get a general idea of where it landed on its first and second bounce. Then I looked at the talcum powder. It showed peaks in the craters (in the talcum powder) and so I measured the distance from the peak in the crater caused by the first bounce and the one caused by the second bounce. 7. I recorded my results. 8. I swept the talcum powder after each bounce. 9. I repeated steps 2-7 5 times using the same tennis ball. 10. I repeated steps 2-8 using a different tennis ball. 11. I repeated steps 2-9 with 5 different temperatures. They were 7oC, 11 oC, 16 oC, 21 oC, and 26 oC. I was not able to get the room to 6oC, so I measured 7oC instead. 12. I varied the temperature by using heaters for heat up the space and by getting up early in the morning to do the low temperatures. 13. The fixed variables are listed below. 14. I processed the results by averaging the results for each ball and then for all 3 balls. graphing temperature against distance travelled. A method describing the independent variable with a valid range, the accurate measurement of the dependent variable and the control of most other variables. Picture 1: Ramp for ball Fixed variables (Potential Errors) Temperature fluctuation during an experiment Barometric pressure Tennis ball wearing out Talcum powder on ball Brand of talcum powder Brand and age of tennis ball Tennis ball Mark on tennis ball Even start Talcum powder consistency How to minimize effect of these Not much of a problem, but at very low temperatures your body heat can rise the temperature in a small room. So if you observe the temperature rising leave the room for a short time to let it cool down. This is a tricky one but on a clear day (without a storm approaching) it will not change substantially so do all tests on a clear day within 24 hours of each other. Use good tennis balls of same brand and do the colder temperatures first. Wipe the ball clean after each trial with a dry towel. Use same brand. Use same brand of new balls. Mark each tennis ball and get individual results for each one. Do the mark as small as possible and in biro, not anything that is likely to change the flight path of the ball. Start each ball with the same object eg block of wood at same height (62.4 cm) Use the same brush and try to keep the talcum powder to as thin and as even as possible. A table showing: - how most other variables were controlled - discussion of sources of error RESULTS: The results were averaged. A graph was drawn showing the averaged data which highlighted the aberrant data for one ball at 26oC. This was removed and the averaged data graphed Table showing Results: temperature (oC) vs distance (cm) between 1st and 2nd bounce. weight (g) ball 1 58 temperature Ball 1 . ball 2 55.9 ball 3 56.8 Ball 2 Ball 3 7 7 7 7 7 147 142 149.5 152 145 140.5 141 140 144.5 136.5 147.5 144 150 141.5 154 11 11 11 11 11 150 144.5 152 153.5 146 143 142 137.5 147 145.5 147 149.5 145.5 145.5 150 16 16 16 16 16 149 151 154 150.5 150 145 144 143 147 145 147 152 146.5 146.5 144.5 21 21 21 21 21 151.5 153 156 148 149.5 148 150 151.5 147.5 145.5 148 151 146 147.5 153 26 26 26 26 26 139 136 146 135 146 149 152 145 150 152 150 145.5 153.5 149.5 150 Average Average Average Average Ball 1 Ball 2 Ball 3 all balls 150.6 140.5 148.8 146.6 152.6 146.3 149.4 149.4 152.9 146.1 149.8 149.6 154.4 150.6 151.6 152.2 145.2 152.2 152.4 152.3 Sufficient, reliable data consistent with the final method, recorded systematically with appropriate precision. Appropriate treatment of extremes of data Graph 1: Disrance (cm) Average distance balls bounced at different temperatures 156 154 152 150 148 146 144 142 140 138 Average Ball 1 Average Ball 2 Average Ball 3 0 10 20 30 temperature in degrees Centigrade Graph 2: Average distance balls bounced at different temperatures with aberant data removed 160 Average Ball 1 Average Ball 2 Average Ball 3 Linear (Average Ball 2) Linear (Average Ball 3) Linear (Average Ball 1) 155 150 145 140 135 0 5 10 15 20 25 temperature in degrees Centigrade Graph 3: 30 Average distance of bounce for all balls vs Temperature 154 Distance (cm) 153 152 Average of all balls 151 150 Linear (Averag e of all balls) 149 148 147 146 0 5 10 15 20 25 30 Temperature in degrees Centigrade Processing of data using graphs and/or other techniques INTERPRETATION: There was a definite relationship between temperature and the distance that the ball bounced, in that the higher the temperature the greater distance the ball bounced. This relationship was true for all the balls that were used, except for one ball at 26oC. I decided to eliminate this aberrant data. Interpretation identifies trend in processed data CONCLUSION: My hypothesis stated that if temperature is increased and all other factors remain the same, then tennis balls would bounce further. My processed data shows that this is true for the 3 tennis balls that I tried. The increase in temperature expands the gases inside the tennis ball, which move around more inside the ball. As the molecules move more they strike the inner surface of the ball and apply more pressure to it. Balls with more air pressure in them bounce better because air, when compressed, will uncompress (spring back) with little or no energy loss. A ball that has higher air pressure in it will not squash as much during the collision, and so less energy will be lost. Therefore the ball will bounce further which is what my investigation showed. Valid conclusion related to the purpose of the investigation. Further explanation of the relevant science ideas DISCUSSION: I was satisfied that I had controlled as many fixed variables as I could. Doing the experiment in an aluminium garage meant that I was satisfied that I kept the temperature constant for each test. However, there are certainly some things that I could do to improve my experiment. Reliability. The data is very reliable because I controlled as many variables as I could. I used tennis balls because they would not lose air as fast as other hollow balls. Validity. This was a valid method although it differed from the methods that I had researched. I used this method as I felt that I could get more accurate results seeing that I was doing this investigation on my own. Limitations. There were limitations to this investigation. - At very low temperatures your body heat can raise the temperature in a small room. So if the temperature rose I would leave the room for a short time to let it cool down. - A sudden decrease or increase in barometric pressure would change the pressure in a ball. I did this experiment on the same day when the weather was not changing. - Measuring the length bounce meant that I did not take into account the height of the bounce. I decided that this was still a valid experiment and that it was not necessary to measure the bounce. Future Investigations. - I would be interested to try the same experiment using different types of hollow balls to see if this trend was true for other hollow balls - I could have tried more of the same brand of tennis ball to see if the aberrant data at 26oC for one ball was true for other balls at that temperature or genuine aberrant data. - I would like to try the method of just dropping the ball from a certain height and measuring the height of the bounce. It would be interesting to compare the 2 sets of data and validate my method that way. - I did consider working out the kinetic energy lost but I decided that these results showed a sufficient trend and proved the hypothesis. Discussion of reliability of data, discussion of sources of error, ideas for future investigations. Justification for final method found in the trialling. BIBLIOGRAPHY: http://wings.avkids.com/Curriculums/Tennis/hotcold_summary.html http://www.phys.virginia.edu/Education/outreach/8thgradesol/Effectoftemperature.ht m http://www.picotech.com/experiments/squash_ball/