Lab 8: Relating Solubility and Temperature
advertisement

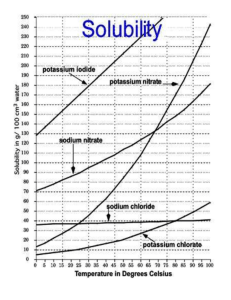
Lab 5: Relating Solubility and Temperature Purpose: To determine the effect of temperature on the solubility of a substance in water and to become proficient at using spreadsheets to graph. Method: Every lab group will be given two temperatures to heat or cool the water to. Then students will add the chemical compound until the water is saturated and record the mass of the chemical compound used in the solution. Vocabulary: solubility Hypothesis: Predict how temperature will effect the solubility of Potassium Nitrate and Sodium Chloride. Materials: 15 grams potassium nitrate 100 mL graduated cylinder Celsius thermometer test tube 1 test tube holder safety goggles Procedure: 1. 2. 3. 4. 5. 6. 7. 8. 9. 10. solute solvent solution saturated water 400 mL beaker Bunsen burner electronic balance ice 10 grams sodium chloride Set up a warm water bath over the Bunsen burner. Obtain 15 grams of potassium nitrate. Measure and record the mass of the potassium nitrate including the beaker. Pour 10.0 mL of water into one test tube. Warm the test tube in the hot water bath until the first temperature assigned to you by your teacher is reached. Try to maintain this temperature throughout the next step. Pour a small amount of potassium nitrate into the test tube. Stir carefully. If the potassium nitrate dissolves completely, add a little more. Continue until no more dissolves and a few small grains settle to the bottom of the test tube. If your solution is no longer at the target temperature, reheat the solution and repeat step 3. Record the exact temperature of the solution. Find and record the mass of the remaining potassium nitrate including the beaker. Determine the amount of potassium nitrate you used by subtracting this amount from the original mass. Repeat the procedure with another 10.0 mL of water. However, this time cool the water in ice until the second temperature assigned to you by your teacher is reached. Again determine the amount of potassium nitrate that dissolves in the water. Record the exact temperature. Record your information in the computer to compile all the information obtained by the class. In this way, you will find out how much potassium nitrate dissolves in 10.0 mL of water over a wide temperature range. Check with the teacher to see if there is a temperature whose data doesn’t make sense and needs to be redone. Repeat the above procedures using the sodium chloride instead of the potassium nitrate. Analysis: Graph your results in lab book. You should have two curves on one graph, one for the potassium nitrate and one for the sodium chloride. Conclusions: 1. 2. 3. 4. 5. 6. What effect does temperature have on the amount of potassium nitrate that can be dissolved in a given amount of water? From the experimental graph, predict how much potassium nitrate would dissolve in 10.0 mL of water at 60 degrees Celsius. From the experimental graph, predict how much potassium nitrate would dissolve in 100.0 mL of water at 60 degrees Celsius. From the experimental graph, predict what temperature 10.0 mL of water would have to be heated to completely dissolve 12 grams of potassium nitrate. If the temperature of a saturated solution of potassium nitrate were to drop, what would be noticed? If 10.0 mL of saturated solution of potassium nitrate were to cool from 60 degrees to 10 degrees Celsius, how much potassium nitrate would be found on the bottom of the test tube? 7. 8. 9. Based on the experimental graph, how much potassium nitrate would dissolve in 10.0 mL of water at 90 degrees Celsius? Compare the solubility curve of potassium nitrate and sodium chloride. Attach the actual solubility curve in your lab book. Number and title the graph. Look at the actual solubility curve for potassium nitrate and sodium chloride and calculate the % error for the temperatures your group measured for both compounds. Remember that the curve is for 100 mL of water and you used 10 mL of water.