Osmosis and Water Potential Lab
advertisement

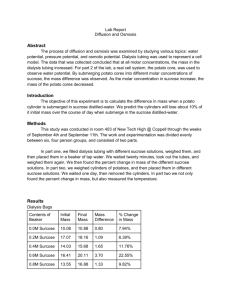
AP Biology Diffusion and Osmosis in Model Systems In parts 1 and 2 of this lab, you will have the opportunity to investigate the processes of diffusion and osmosis in model membrane systems. You will also investigate the effect of solute concentration on water potential as it relates to living plant tissues. Objectives: At the completion of this AP laboratory, you should be able to: 1. Describe the mechanisms of diffusion and osmosis. 2. Describe how solute size and molar concentration affect the process of diffusion through a selectively-permeable membrane. 3. Describe the relationship between solutions that are hypotonic, hypertonic, or isotonic. 4. Design an experiment to demonstrate water potential. 5. Relate osmotic potential to solute concentration and water potential. 6. Describe how pressure potential could affect the water potential of a solution. 7. Describe the effects of water gain or loss in animal and plant cells. 8. Calculate the water potential of living plant cells from experimental data. Background Information Part I. Osmosis. In this part of the lab, you will use dialysis tubing filled with different molarities of sucrose to investigate the relationship between solute concentration and the movement of water through a selectively permeable membrane (the process of osmosis). When a dialysis bag containing a sucrose solution is placed in fresh water, the bag accumulates water as a result of osmosis. Because there is a higher concentration of water outside the bag than inside the bag, water diffuses into the bag. The water outside the bag is said to be hypotonic to the solution in the bag; the solution inside the bag is hypertonic relative to the water outside the bag. Water always moves through a selectively permeable membrane (sucrose cannot move) from hypotonic to hypertonic solutions. In theory, if you were to add solute to the water outside the bag, you could decrease the water concentration out there. It should be possible to add just the right amount of solute to the water so that the concentration of dissolved substances outside the bag is the same as the concentration of dissolved substances inside the bag.. Such solutions are said to be isotonic, and no net movement of water will occur. If you were to continue to add solute to the water outside the bag, you would decrease the concentration of water outside the bag until the solution outside became hypertonic to the solution inside. Then, the net movement of water would be in the opposite direction: from inside to outside, and the bag would shrink. Part 2. Determining the Water Potential of Potato Cells. In this part of the lab, you will use cores of potato tissue placed in different molar concentrations of sucrose in order to determine the water potential of potato cells. First, however, let’s explore what we mean by the idea of water potential! In animal cells, movement of water into and out of the cell is influenced by the relative concentration of solute on either side of the cell membrane. If water moves out of the cell, the cell will shrink, (crenulate), and if the water moves into the cell it will swell, and may even burst (cytolize). In plant cells, the presence of a rigid wall prevents cells from bursting as water enters the cells, but pressure eventually builds up inside the cell and affects the process of osmosis. In predicting which direction water will move through living plant tissues, a quantity known as water potential is used. Water potential, abbreviated by the Greek letter SI refers to the potential energy of water. It has many components, but the two on which we will focus are osmotic potential which expends on solute concentration, and pressure potential ,which results from the exertion of pressure (positive or negative) on a solution. We express this as: ψ water potential = ψp = pressure potential ψs + + osmotic potential Water will always move from an area of higher water potential (higher potential energy) to an area of lower water potential (lower potential energy). Stated another way, water potential measures the tendency of water to leave one place in favor of another place. The water potential of pure water in a beaker open to the atmosphere is “0” ( ψ = 0 ), because both the osmotic and pressure potentials are taken to be zero ( ψs = 0; ψp = 0). An increase in positive pressure raises the pressure potential, and therefore raises the water potential. The addition of solute to water lowers the osmotic potential (makes negative), and therefore lowers the water potential. Therefore, any solution at atmospheric pressure (ψp = 0) will always have a negative water potential. For example, a 0.1 M sucrose solution at atmospheric pressure (ψp = 0) has an osmotic potential (ψs) of –2.3 bars due to the solute, and thus a total water potential of –2.3 bars ( 0 + -2.3 = -2.3). A bar is a metric unit of pressure, measured with a barometer, that is about the same as one atmosphere. When a solution, such as that inside a potato cell, is separated from pure water by the selectively permeable cell membrane, water will move by osmosis from the surrounding area where water potential is higher (ψ = 0 ) into the cell where water potential is lower due to its dissolved solutes (ψ is negative). In a case where the solute cannot leave the cell, the movement of water into the cell causes the cell to swell, and the cell membrane pushes against the cell wall. This creates positive turgor pressure in the cell. Eventually, enough positive turgor pressure builds up to oppose the more negative osmotic pressure of the cell. This will continue until the water potential of the cell equals the water potential of the pure water outside the cell (ψ cell = ψ outside the cell = 0 ). At this point, a dynamic equilibrium is reached and NET movement of water will cease. If you add solute to the water outside the potato cells, you decrease the water potential of the solution surrounding the cells. It should be possible to add just the right amount of solute to the water so that the water potential outside the cell is the same as the water potential inside the cell. Therefore, net water movement will cease. At this point, the water potential of the solution is equal to the water potential of the turgid potato cells. (It is this information that you will use to calculate the water potential of cells in the potato cores, which have been soaking in different molarities of sucrose.) Eventually, if enough solute is added to the solution in the beaker, the water potential of the cell will be greater (less negative) than the water potential of the solution in the beaker because of the turgor that still exists within the cells. Water will diffuse out of the potato cells in response to a pressure gradient, from the area of higher water potential (inside) to the area of lower water potential (outside). If enough water was lost, the cell membrane would shrink and fall away from the cell wall, and the cell would become plasmolyzed. By weighing some potato cores and immersing them in different sucrose solutions, then re-weighing, you should be able to pinpoint the sucrose solution which corresponds to the water potential of the potato tissue! Lab: Diffusion and Osmosis in Model Systems Part 1 – SUGAR WATER Objective: To determine the sucrose concentration of six unknown sugar water solutions using osmosis. Materials: Dialysis tubing Sucrose solutions (0 M, 0.2 M, 0.4 M, 0.6 M, 0.8 M, 1.0 M) Cups Distilled water Procedure: You will be responsible to designing you own procedure. Make sure you use enough detail that would allow someone to repeat your experiment. Here is some help to get you started: Dialysis tubing (which allow water to penetrate, but not sucrose): 1. The tubing must be cut into strips about 15 cm in length and soaked in fresh distilled water before you can open it. 2. Open one of the strips by rubbing it between your fingers. Then, twist one end of the tubing, fold over a small portion of the twisted section and firmly tie it closed with a piece of string to make a water-tight seal. 3. Make sure you record the amount of solution you put in each bag. 4. Remove most of the air from the bag by drawing the dialysis bag between two fingers. To close the bags, tie a knot at the other end, thereby sealing the solution within the bag. BE SURE that you have left 1.5 to 2 times as much empty space as that taken up by the volume of the solution in the bag. (This leaves enough unfilled space within the bag to accommodate the possible accumulation of water.) 5. Carefully rinse off and blot dry the outside of each dialysis bag. Measure and record the initial mass of each of your bags. 6. Place each bag in one of the beakers of distilled water and label the beakers to indicate the molarity of the solution in the dialysis bags. 7. Let stand overnight. Part 2 – WATER POTENTIAL OF POTATO CELLS Objective: To determine the sucrose solution from PART A that is iso-osmolar to the potato (the same sucrose concentration) an calculate the water potential of the potato cells. Materials per group: Fresh potato cubes Balance Cups Sucrose solutions (0 M, 0.2 M, 0.4 M, 0.6 M, 0.8 M, 1.0 M) Procedure: 1. Design an experiment to determine which of the six sucrose solutions is iso-osmolar to the potato. Graphs To Include in Your Report 1. Graph 1: Table 1 (Part 1) data. 2. Graph 2: Table 2 (Part 2) data. Data Analysis, Water Potential of the Potato Cells 1. Determine the iso-osmolar concentration of the potato tissue and the sucrose solution by examining Graph 2. Note the point on the curve corresponding to zero change in potato mass (y-axis); drop a perpendicular line to find the corresponding point on the x-axis. This point will represent the concentration of sucrose iso=osmolar to the potato issue. At this concentration there is no net gain or loss of water from the tissue. (From graph) Iso-osmolar concentration = ____________M. 2. The osmotic potential (ψ) of iso-osmolar sucrose solution can be calculated from the molarity of the solution by applying this formula: ψ = iCRT where: i = ionization constant (for sucrose, we can use a value of 1 since sucrose does not ionize in water). C= the iso=osmolar concentration (usually expressed in molality; read off the graph) R= pressure constant (handbook value R = 0.0821 bar/mole deg K for water) T= temperature, degree Kelvin (273 + degrees C) of solution. What is the osmotic potential of the sucrose solution found (by your graph) to be iso-osmolar to the potato tissue? 3. Since the pressure potential (ψp) of our solutions was zero, we can calculate the water potential of the solution from the equation for water potential given in the pre-lab. The water potential of the solution at equilibrium will be equal to the water potential of your potato cells. What, then is the water potential of your potato cells? Questions (Discussion Section) 1. You have calculated the water potential of your potato cells. Earlier you learned that pure water at standard pressure and temperature has a water potential of 0. If you threw your potato into a jar of pure H20, would the potato shrink, or swell? Explain. 2. We said the water potential (ψ) of the potato cells is equal to the water potential of the solution, at equilibrium. Is the osmotic potential (ψs) of your potato cells the same as that of the solution? Why or why not? 3. If a potato cylinder is allowed to dehydrate by sitting in the open air, would the potato cells’ water potential become higher or lower? Explain. Data Table 1, Part 1 Data Contents of Dialysis Bags Initial Mass Final Mass Percent Change in Mass Class Ave. % Change Initial Mass Final Mass Percent Change in Weight Class Ave. % Change 0.0 M Sucrose 0.2 M Sucrose 0.4 M Sucrose 0.6 M Sucrose 0.8 M Sucrose 1.0 M Sucrose Table 2; Part 2 Data Sucrose Solutions Soaking Potato Cores 0.0 M Sucrose 0.2 M Sucrose 0.4 M Sucrose 0.6 M Sucrose 0.8 M Sucrose 1.0 M Sucrose HINT: % Change = Final Mass – Initial Mass Initial Mass