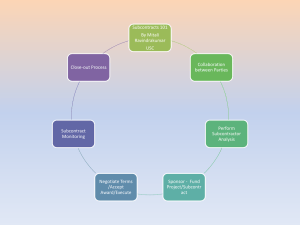
EVALUATION PLAN: TELEPHONE SURVEY SAMPLE
advertisement

Florida International University Office of Research & Economic Development Subcontract/Amendment Request Form Office of Sponsored Research & Economic Development Subcontracting Group To: From: Re: Subcontract/Amendment Request Date: Thursday, March 03, 2016 Note: Please return the completed and signed form, with attachments, to my attention for processing. Appendices must be typed attachments. Once the subcontract/amendment is complete it will be returned for your review before forwarding to the subcontractor for execution. 1. Project ID: Principal Investigator: 2. Document type: New Subcontract 3. 4. Sponsor: Amendment to existing subcontract If this is an amendment request, please explain what in the original subcontract needs to be modified. Name of the Subcontractor (University/Organization/etc.) 5. Period of performance for the subcontract/amendment 6. Contact information for Subcontractor PI From: Name: To: Title: Address: Telephone: Fax: Email: 7. Contact information for Subcontractor Contract Administrator Name: Title: Address: Telephone: Fax: Email: 8. To whom at Subcontractor should FIU send the completed subcontract/amendment? Name: Title: Address: Telephone: Fax: Email: 1 4/21/2014 Florida International University Office of Research & Economic Development 9. Statement of work for the subcontractor (please attach typed statement of work as Appendix A and be as complete as possible) 10. Budget or deliverable schedule for the subcontractor's work (please attach budget as Appendix B) 11. Termination notice for the subcontract: 30 or 60 days? 12. Subcontract above 75K requires a Bid Exemption Certification (online form: http://research.fiu.edu/) 13. Conflict of Interest: Is there any existing or potential conflict of interest relating to this subcontract and the research project? 30 days 60 days Yes No See the Conflicts of Interest in Research policy, #2370.005, at http://policies.fiu.edu/files/572.pdf for more information. Note that disclosures are required from all FIU personnel, whether they are full or part-time employees or volunteers, if such persons have a conflict of interest as relates to the research project. This includes, but is not limited to, volunteer faculty, adjuncts and students. If you answered YES above, the applicable FIU individual must complete the Outside Activity/Conflict of Interest Report at http://hr.fiu.edu/index.php?name=conflict_of_interest. The disclosure will be reviewed to determine if the conflict can be managed, reduced or eliminated so that the subcontract may proceed. 14. IRB, IBC or IACUC Approvals: Will there be any studies carried out by the subcontractor, which involve human subjects (including surveys or data analysis), animals or recombinant DNA molecules? No, none apply Yes, human subjects. Yes, animal use. Yes, recombinant DNA. If any have been checked “yes,” does the attached scope of work accurately reflect what subcontractor will do in this regard? Yes No Please note that IRB/IACUC/IBC approval from both FIU and the subcontractor are required. The subcontract and/or amendment will not be executed by FIU until satisfactory documentation is received to meet this requirement. 15. Does the sponsor award contain any conditions pertaining to export control s (e.g., foreign national prior approval, publication limitations) or other confidentiality requirements? Yes No If you answered YES above, list the sponsor award sections that address those requirements: 16. Does the sponsor award require prior sponsor approval of the subcontractor or the subcontract agreement? Yes No If you answered YES above, list the section of the sponsor award the addresses that: Attach a copy of the written approval from the sponsor for this subcontract agreement. 17. Previously Executed Agreements: Is this subcontract related to a previously executed confidentiality of other agreement with the subcontractor? Yes No If yes, please identify which prior agreement: PI Signature: ___________________________________ Date: ___ _______________ Name (typed): Phone: 2 4/21/2014 Florida International University Office of Research & Economic Development Email: FOR ORED USE ONLY Export Controls: ___________/_______________/____________ Restricted Parties Search (RPS) completed on: RPS results attached to this memo. IRB/IACUC/IBC Approvals: If subcontract SOW involves human subjects, animal use or Recombinant DNA, verify that IRB/IACUC/IBC approval from BOTH the subcontractor and FIU are in place. Subcontractor IRB approval No: _____________________ received FIU IRB approval No: _____________ in place Subcontractor IACUC approval No: _____________________ received FIU IACUC approval No: _____________ in place Subcontractor IBC approval No: _____________________ received FIU IBC approval No: _____________ in place ORED Representative Signature: ______________________________ Date: _______________________ Pre-Award: If subcontract scope of work requires IRB, IACUC and/or IBC approval and such approval(s) from both the subcontractor and FIU cannot be verified, route to ORI for review of IRB, IACUC and/or IBC requirements. ORI, please check as applicable: Appropriate IRB/IACUC/IBC approval is in place for subcontractor’s work. Subcontract may proceed to execution. Subcontract may not be executed. IRB/IACUC/IBC has not approved subcontractor’s involvement in the study. __________________________________________ Signature Print name Date ORI Comments: 3 4/21/2014