Cells – Living Factories
advertisement

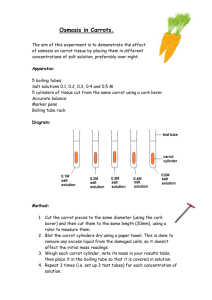
Page 1 – Demonstrating Osmosis in Carrot Tissue Demonstrating Osmosis in Carrot Tissue Background Osmosis is the diffusion of water through a semipermeable membrane. The cell membranes of carrots exhibit this selective permeability. These membranes allow water and small nonpolar molecules to pass freely into and out of the cells of the carrot according to the surrounding concentration gradient. As you have already learned, water moves from areas of low solute concentration (high water concentration) to areas of high solute concentration (low water concentration.) Therefore, if carrot cells are placed in a solution with a higher or lower solute concentration, then water will move across the cell membranes of the carrot cells until the cells and the solution have reached equilibrium. Water will move at different rates depending upon the strength of the concentration gradient that exists between the carrot cells and the solution. The stronger the gradient, the faster the water will move. In this laboratory, you will explore how the concentrations of various solutions cause carrot cells to either take up or lose water. You will be placing carrot slices in each of the following four solutions: pure water, and 1%, 2%, and 3% NaCl (salt) solutions. After a short period of time you will compare the mass of the carrot slices to their original mass to determine if the carrots either gained or lost water through osmosis. At the conclusion of this laboratory you will analyze your data to estimate the solute concentration of carrot tissue. Objectives In this experiment, you will Understand how the concentration gradient between cells and their environment can cause water to move by osmosis. Use an electronic balance to calculate the percent weight gain or loss of carrots placed in a solution of known tonicity (strength). Determine which solution concentration(s) will cause carrot cells to gain water? Lose water? Analyze percent weight gain/loss data to estimate the solute concentration of carrot tissue. Pre-Lab Questions 1. What is osmosis? 2. What is a concentration gradient? How does the concentration gradient affect the rate at which osmosis occurs? 3. In which solutions do you think the carrot cells would gain water by osmosis? What term would you use to describe these solutions? 4. In which solutions do you think the carrot cells would lose water by osmosis? What term would you use to describe these solutions? 5. What solute concentration do you think will be isotonic to carrot tissue? Page 2 – Demonstrating Osmosis in Carrot Tissue Lab Report Hint: Using your answers from Pre-Lab questions 3-5, create a hypothesis about what will happen to the carrot cells in each of the four solutions. Materials Each group will need the following: raw carrot electronic balance blade or knife 4 petri dishes distilled water grease pencil 1%, 2%, and 3% NaCl solutions paper towels calculator Safety Because of the use of sodium chloride solutions, goggles and lab aprons should be worn at ALL times. Students will also be using blades to slice carrots. Care should be taken to avoid cutting fingers or other body parts. Use caution when handling glassware as it can break easily and cut you. Procedure 1. Using the blade, carefully slice the carrot into discs. You will need 20 discs that are 2-3 mm thick. Be careful to cut away from you and keep fingers out of the way of the blade. 2. Place a paper towel on the weighing dish of the electronic balance and tare the balance. 3. Place 5 carrot discs on the paper towel and weigh them as a group. Record the weight of these discs in the table provided under “mass at start.” Spread the five discs flat in a single layer in a petri dish. 4. Repeat steps 2-3 for the remaining carrot discs. 5. Label one petri dish “0% NaCl.” Label the remaining dishes “1% NaCl,” “2% NaCl,” and “3% NaCl.” This refers to the percentage of NaCl (sodium chloride or “salt”) by weight that is present in each solution. 6. Fill the petri dish labeled 0% NaCl with enough distilled water to just cover the carrot slices. 7. Fill the other petri dishes accordingly with the sodium chloride solution that corresponds to their label. Set all petri dishes aside. Allow the carrot slices to soak in the solutions for at least 15 minutes, and then proceed to the next step. 8. Drain the liquid from each petri dish into the sink. Gently blot the carrot discs with a paper towel. Be careful to not mix them up. 9. Re-tare the balance and re-weigh each set of discs. Record the result under “mass at end” in the data table. 10. Feel the texture of your carrot slices. Note your observations and any differences between groups of slices in the data table. 11. Discard the carrots in the garbage. Rinse and dry the petri dishes and leave them on a paper towel at your station. Page 3 – Demonstrating Osmosis in Carrot Tissue 12. Using a calculator, find the percent weight change for each group of carrots. This can be determined by using the following formula: (mass at end - mass at start) x 100 = percent weight change mass at start Record these percentages in your data table. 13. Collect the percent weight change values of other lab groups and find the mean (average) weight change for each solution. Record this in your data table. Data Table SOLUTIONS 0% NaCl Strength Pure water Mass at start (g) Mass at end (g) Texture (soft or firm) Percent Weight Change (%) Percent Weight Change (%) of other groups Mean (Average) Weight Change (%) Tonicity of Solution (compared to carrot cells) 1% NaCl 2% NaCl 3% NaCl (increasingly stronger salt (NaCl) solutions) Page 4 – Demonstrating Osmosis in Carrot Tissue Post-Lab Questions: 1.) Why use carrot slices instead of large chunks of carrot? 2.) Why is it important to use the percent weight change when determining the effects of osmosis? Why not just evaluate the change in weight? 3.) What does it mean if the percent weight change is a negative number? 4.) Create a line graph of solution concentration vs. mean (average) weight change of carrot slices. Your graph should meet the following requirements: It should be done on graph paper or using a spreadsheet program (such as Microsoft Excel). The independent variable (solution concentration) should be on the x-axis. Spread the values evenly along the axis and include the proper units in the axis label. The dependent variable (mean weight change) should be included on the y-axis. Space these values appropriately along the axis and include the proper units in the axis label. Note: This axis may need to extend both above and below the x-axis to fit negative values. Include an appropriate title for your graph. A line of “best fit” plotted through your data points. The graph should be included in the “Analysis” section of your laboratory report. 5.) Based on your results, what might be the concentration of solutes in the cytoplasm of the carrot cells at the start of this experiment? 6.) What do you think will happen to the carrot slices if you leave them in the solutions overnight? 7.) What can you say about the reliability of this experiment? Hint: How close were other group’s results to yours? 8.) Explain any inconsistencies in your data. Could an error on the part of a lab group have resulted in these inconsistencies? If so, how? Lab Report Hint: Use your answers from the Post-lab questions above to write your Analysis section. References: Pupil’s experiments with osmosis in carrot tissue. Retrieved on November 18, 2002 from <http://www.biotopics.co.uk/life/carrot.html>