File - MHS

L
AB
: S
OLUBILITY AND
T
HERMODYNAMICS
I
NTRODUCTION
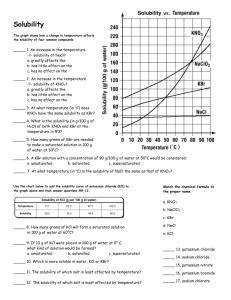
In this experiment, we will set out to investigate the thermodynamic quantities associated with the dissolution of potassium nitrate, KNO
3
, in water.
KNO
3
(s)
K + (aq) + NO
3
(aq)
Potassium nitrate dissolves in water like all nitrate salts. Energy changes arise in the dissolution process due to breaking ionic bonds and the formation of interactions between the ions and the solvent. Plausible arguments can be made for classifying the dissolution of the salt as a physical or chemical process.
We know that energy changes are associated with all chemical and physical processes due the changes in kinetic and potential energy as the ionic bonds are broken and the interactions of the ions and solvent follow. In this lab, you will endeavor to determine the thermodynamic quantities associated with the dissolution of KNO
3
: the enthalpy change, the entropy change and the Gibbs free energy change.
MATERIALS
Solid KNO
3
Water bath
100 mL graduated cylinder with removable plastic bottom.
PRE-LAB QUESTIONS :
1.
Read the procedure in this lab and design data tables to collect the necessary data and perform important calculations. Put the procedure and tables in your lab notebook. (Suggest one table for measured data and another for calculated data.)
2.
What variables must be placed on the x and y-axes when graphing the solubility of KNO
3
?
3.
Draw a graph based on your answer to question one and sketch your predicted slope?
4.
Draw a particulate diagram of the species that exist at the saturation point.
5.
Write an equation for the dissolution of KNO
3
and write the equilibrium expression (Ksp). (Hint, since KNO
3
is a solid, it will NOT be included in the equilibrium expression. Only the ions are included.)
6.
Predict the sign of ∆S and ∆H for the dissolution of KNO
3
.
P ROCEDURE
CAUTION: Potassium nitrate is a strong oxidizer and skin irritant. Avoid contact with skin, eyes and mucous membranes. Avoid shock, heat, and contact with combustible materials.
1. Weigh 20.0 g of potassium nitrate and transfer it to a 100 ml graduated cylinder (without the plastic bottom).
2. Add 15 mL of water and heat the test tube in a boiling water bath, stirring until all of the KNO
3 has dissolved.
3. Determine and record the volume of the potassium nitrate solution.
4. Remove the test tube with the potassium nitrate from the water bath and allow it to cool while slowly stirring the solution with a thermometer.
5. Record the temperature when crystals first appear.
It is assumed at this temperature that the system is at equilibrium, and it is possible to calculate the concentration of the ions.
6. Add 5 mL of water to the test tube and warm the mixture in a water bath again until the solid has all dissolved. Determine the solution volume as before and record it.
7. Cool the solution slowly and record the temperature at which crystals first appear.
8. Repeat the cycle (steps 6 and 7) adding 5 mL of water, heating and cooling until crystals appear near room temperature. This should take 5 to 7 determinations.
Post Lab
1. Construct a solubility curve for KNO
3
.
2. Calculate the equilibrium constant at each temperature for the dissolution of KNO
3
. As noted in the pre-lab questions Ksp = [K + ][NO
3
]. You do know the concentration of both ions in solution (i.e. they are the same as the molar solubility in this case). Show your work and include these values in a calculated data table.
3. Based on your calculated value your equilibrium constant, what do you predict about the magnitude of the value of ∆G, Gibbs free energy, at the different temperatures?
4. Calculate the numerical value of ∆G for each of your temperatures (remember that
G = -RTlnK). Show your work and include these values in a calculated data table.
1
5. Construct a graph of the natural logarithm of K, ln K, versus for each individual temperature.
T
6. What value can be determined from the slope of this graph? Explain and determine its value.
1
(Hint: ∆G = -RTlnK and ∆G = ∆H - T∆S, therefore, -RTlnK = ∆H - T∆S or lnK = ( ) + )
T
7.
You determined the ΔH value for the following process by plotting the data. The accepted ΔHfº,
ΔGfº and ΔSº values for the process are given below. Calculate the ΔHrxn, ΔGrxn and ΔSrxn from these values (show all work). Compare to your empirical (laboratory) values and explain any disagreement.
KNO3(s)
ΔHfº (kJ/mol) -492.7
ΔGfº (kJ/mol)
ΔSrxn(J/mol·K)
-393.1
+132.9
8.
How does the sign on ΔS “fit” this process? Is this process entropy or enthalpy driven – or both?
Explain
==> K +
(aq)
+
-251.2
-282.3
+102.5
NO - 3
(aq
-206.6
-110.6
+146.4
)
Reference:
1. Silberman, Robert G., SUNY at Cortland N.Y., filtrates and Residues, Journal of Chemical Education, May,
1996, p. 426.