BIO-302 Midterm 1 2007
advertisement

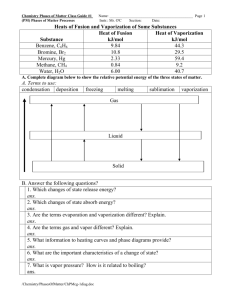
Bio301, Biochemistry II, Midterm Examination 1, March 26, 2007 Fill in the blanks: 1. The specific transport of a species down its concentration gradient is referred to as_............................... 2. Membrane pums are energy …………………………….. 3. In ……………………………………………. a phosphoryl group is transferred from ATP to a specific aspartate residue. 4. Cardiac steroids such as digitoxigenin inhibit the……………………………… 5. The lactose permease transports lactose into the cell along with a ………………………… 6. The acetylcholine receptor is an example of a……………………………-gated channel. 7. ………………………………………………… is a primary messenger that is released by the adrenal gland. 8.………………………………… is another name for the seven-transmembrane helix receptors. 9. The and subunits of heterotrimeric G proteins are anchored to the cell membrane by being covalently linked to this molecule (……………………………………….). 10. This enzyme (…………………………….) becomes active when bound to Ca2+ and diacylglycerol. 11. This protein (…………………………………….) binds to calcium ions and serves as a Ca2+ sensor in eukaryotic cells. 12………………………………………………..is a secondary messenger and is abbreviated IP3 13…………………………………………is the membrane protein that catalyzes the conversion of ATP to camp 14. The cytosolic side, or β subunit, of the insulin receptor is a ……………………………kinase. 15. The binding of IP3 to the IP3 receptor results in the release of ……………………………from the endoplasmic reticulum. 16………………………………………………… binds to β-andrenergic receptors 17. The Acetyl group is attached to Coenzyme A by a ……………………………………….bond. 18…………………………… reactions form new bonds by using free energy from ATP cleavage. 19. Activated carriers……………………..and…................................... and…………………. contain adenosine diphosphate units. 20. G proteins are converted from the GTP bound form to the GDP bound form by nucleotide…………………… they are converted from the GDP bound form to the GTP bound form by………………………………… . 21. How does the potassium channel maintain selectivity for potassium versus sodium ions? A) The ion size is the determining factor. B) The size of the ion and associated waters relative to the pore size is the determining factor in channel selectivity. C) Dehydration of the potassium ion is compensated energetically by interactions with oxygen atoms in the selectivity filter, which is not possible with sodium ions. D) All of the above. E) None of the above. 22. When a molecule moves from a concentration of 104 M to 102 M, is the process spontaneous, at equilibrium, or does it require an input of energy? A) at equilibrium D) All of the above. B) input of energy required E) None of the above. C) spontaneous 23. What are the two messenger products formed by cleavage of PIP2? A) diacylglyercol and inositol 1,4,5-triphosphate B) diacylglyercol and inositol 1,3,5-triphosphate C) diacylglyercol and inositol 1,3-diphosphate D) diacylglyercol phosphate and inositol 1,4,5-trisphosphate E) None of the above. 24 How is calmodulin activated? A) by binding of both calcium and potassium B) by binding Ca2+ when the cytosolic concentration is greater than 500 nM C) by binding to a positively charged helix on another protein D) All of the above. E) None of the above. 25 How does the potassium channel maintain selectivity for potassium versus sodium ions? A) The ion size is the determining factor. B) The size of the ion and associated waters relative to the pore size is the determining factor in channel selectivity. C) Dehydration of the potassium ion is compensated energetically by interactions with oxygen atoms in the selectivity filter, which is not possible with sodium ions. D) All of the above. E) None of the above. 26. (10 points) Define action potential, and explain its mechanism in terms of the transient changes in Na and K permeability of the plasma membrane of a neuron. 27. (15 points) Show (by calculation!) that the hydrolysis of a single ATP molecule provide sufficient energy for the process of transporting 3 molecules of Na+ out and two molecules of K+ into the cell under cellular conditions in which Na+ is present 143 mM outside and 14 mM inside the cell, and K+ is present 4 mM outside and 157 mM inside the cell at a membrane potential is -60 mV (inside negative) at 37oC. (Faraday constant is 23.1 kcal.V-1mol-1, and Gas constant is 1.987 cal.mol-1.K-1) 1. The specific transport of a species down its concentration gradient is referred to as _______________________. Ans: passive transport, or facilitated diffusion Section: Introduction, 13.1 2. Membrane pumps are energy ____________________. Ans: transducers Section: Introduction 3. In _________________ a phosphoryl group is transferred from ATP to a specific aspartate residue. Ans: P-type ATPases Section: 13.2 4. Cardiac steroids such as digitoxigenin inhibit the _______________________. Ans: Na+-K+ ATPase pump Section: 13.2 5. The lactose permease transports lactose into the cell along with a ______________. Ans: H+, proton Section: 13.3 6. The acetylcholine receptor is an example of a ____________-gated channel. Ans: ligand Section: 13.4 31. How does the potassium channel maintain selectivity for potassium versus sodium ions? A) The ion size is the determining factor. B) The size of the ion and associated waters relative to the pore size is the determining factor in channel selectivity. C) Dehydration of the potassium ion is compensated energetically by interactions with oxygen atoms in the selectivity filter, which is not possible with sodium ions. D) All of the above. E) None of the above. Ans: C Section: 13.4 When a molecule moves from a concentration of 104 M to 102 M, is the process spontaneous, at equilibrium, or does it require an input of energy? A) at equilibrium D) All of the above. B) input of energy required E) None of the above. C) spontaneous Ans: B Section: 13.1 7. ____________ This is a primary messenger that is released by the adrenal gland. Ans: j epinephrine Section: 14.1 8. ____________ This is another name for the seven-transmembrane helix receptors. Ans: a serpentine receptors Section: 14.1 9. ____________ The and subunits of heterotrimeric G proteins are anchored to the cell membrane by being covalently linked to this type of molecule. Ans: c fatty acids Section: 14.1 10. ____________ This enzyme becomes active when bound to Ca2+ and diacylglycerol. Ans: I protein kinase C Section: 14.1 11. ____________ This protein binds to calcium ions and serves as a Ca2+ sensor in eukaryotic cells. Ans: b calmodulin Section: 14.1 12. _______________________ is a secondary messenger and is abbreviated IP3. Ans: Inositol 1, 4, 5-trisphosphate Section: Introduction 13. ____________ is the membrane protein that catalyzes the conversion of ATP to cAMP. Ans: Adenylate cyclase Section: 14,1 14. The cytosolic side, or β subunit, of the insulin receptor is a ________________ kinase. Ans: tyrosine Section: 14.2 15. The binding of IP3 to the IP3 receptor results in the release of __________ from the endoplasmic reticulum. Ans: calcium ion Section: 14.1 16. _______________________ binds to β-andrenergic receptors. Ans: Epinephrine, adrenaline Section: 14.1 17. What are the two messenger products formed by cleavage of PIP2? A) diacylglyercol and inositol 1,4,5-triphosphate B) diacylglyercol and inositol 1,3,5-triphosphate C) diacylglyercol and inositol 1,3-diphosphate D) diacylglyercol phosphate and inositol 1,4,5-trisphosphate E) None of the above. Ans: A Section: 14.1 18. How is calmodulin activated? A) by binding of both calcium and potassium B) by binding Ca2+ when the cytosolic concentration is greater than 500 nM C) by binding to a positively charged helix on another protein D) All of the above. E) None of the above. Ans: B Section: 14.2 19. How is the hormone-bound activated receptor reset after activation? Ans: The hormone dissociates, and the receptor returns to its initial state. In addition, phosphorylation of specific residues leads to the binding of β–arrestin, which further diminishes its ability to activate G-proteins. 20. In the cell, the hydrolysis of an ATP molecule in a coupled reaction changes the equilibrium ratio of products to reactants by a factor of __________. Ans: 108 Section: 15.2 21. The Acetyl group is attached to Coenzyme A by a _______________ bond. Ans: thioester Section:15.4 22. _____________ reactions form new bonds by using free energy from ATP cleavage. Ans: Ligation Section: 15.4 23. Which activated carriers contain adenosine phosphate units? A) NADH B) FADH2 C) coenzyme A D) a and b E) a, b, and c Ans: E Section: 15.4 20. G proteins are converted from the GTP bound form to the GDP bound form by _____; they are converted from the GDP bound form to the GTP bound form by _____ . a. Nucleotide exchange; nucleotide exchange b. Nucleotide exchange; nucleotide phosphorylation c. Nucleotide hydrolysis; nucleotide exchange d. Nucleotide hydrolysis; nucleotide phosphorylation e. Substrate phosphorylation; nucleotide exchange i. c Describe how metabolic processes are regulated. 24. Explain how a metabolic pathway can contain an energetically unfavorable reaction yet still be occur. Ans: The free-energy changes of the individual steps in a pathway are summed to determine the overall free-energy change. Thus, a step that might not normally occur can be driven if it is coupled to a thermodynamically stable reaction. Section: 15.1 25. If many compounds are common to both anabolic and catabolic paths, how can metabolism be controlled? Ans: The enzymes and their activities can be controlled by the energy charge in the cell. The biosynthetic and catabolic paths are different from each other and may even be located in different compartments in the cell. Thus the two opposing processes can be controlled independently. Section: 15.4 Define action potential, and explain its mechanism in terms of the transient changes in Na and K permeability of the plasma membrane of a neuron. Show (by calculation) that the hydrolysis of a single ATP molecule provide sufficient energy for the process of transporting 3 molecules of Na+ out and two molecules of K+ into the cell under cellular conditions in which Na+ is present 143 mM outside and 14 mM inside the cell, and K+ is present 4 mM outside and 157 mM inside the cell at a membrane potential is -60 mV (inside negative) at 37oC. (Faraday constant is 23.1 kcal.V-1mol-1, and Gas constant is 1.987 cal.mol-1.K-1)