thermal ratio
advertisement

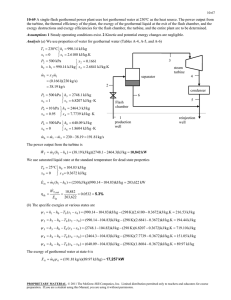
ΕΡ&Σ Π Α Ν Ε Π Ι Σ Τ Η Μ Ι Ο Θ Ε Σ Σ Α Λ Ι Α Σ ΠΟΛΥΤΕΧΝΙΚΗ ΣΧΟΛΗ ΤΜΗΜΑ ΜΗΧΑΝΟΛΟΓΩΝ ΜΗΧΑΝΙΚΩΝ LFM&T ΕΡΓΑΣΤΗΡΙΟ ΡΕΥΣΤΟΜΗΧΑΝΙΚΗΣ & ΣΤΡΟΒΙΛΟΜΗΧΑΝΩΝ Α.Δ. Παπαθανασίου, Αν.Καθ. Λεωφ. Αθηνών-Πεδίον Άρεως-38334 Βόλος - Τηλ. 24210 74094/11- email: fluids@mie.uth.gr - web: http://www.mie.uth.gr/labs/fluids PROBLEM 9-157 Assumptions 1 The air-standard assumptions are applicable. 2 Kinetic and potential energy changes are negligible. 3 Air is an ideal gas with variable specific heats. Analysis (a) For this problem, we 350°C 25°C use the properties of air from EES 5 Combustion software. Remember that for an ideal Heat chamber gas, enthalpy is a function of exchanger temperature only whereas entropy is 3 2 1.2 MPa functions of both temperature and 4 500°C pressure. Sat. vap. 200°C Turbine Compress. Process 1-2: Compression T1 30 C h1 303 .60 kJ/kg T1 30 C s1 5.7159 kJ/kg K P1 100 kPa 1 100 kPa 30°C P2 1200 kPa h2 s 617 .37 kJ/kg s 2 s1 5.7159 kJ/kg.K C h2 s h1 617 .37 303 .60 0.82 h2 686 .24 kJ/kg h2 h1 h2 303 .60 Process 3-4: Expansion T4 500 C h4 792 .62 kJ/kg T h3 h4 h 792 .62 0.82 3 h3 h4 s h3 h4 s We cannot find the enthalpy at state 3 directly. We find h3 = 1404.7 kJ/kg, T3 = 1034ºC, s3 = 6.5699 kJ/kg.K. The solution by hand would require a trial-error approach. Also, T5 350 C h5 631 .44 kJ/kg The inlet water is compressed liquid at 25ºC and at the saturation pressure of steam at 200ºC (1555 kPa). This is not available in the tables but we can obtain it in EES. The alternative is to use saturated liquid enthalpy at the given temperature. Tw1 25 C hw1 106 .27 kJ/kg P1 1555 kPa Tw2 200 C hw2 2792 .0 kJ/kg x2 1 The net work output is wC,in h2 h1 686 .24 303 .60 382 .64 kJ/kg wT, out h3 h4 1404 .7 792 .62 612 .03 kJ/kg wnet wT, out wC,in 612 .03 382 .64 229 .39 kJ/kg The mass flow rate of air is W net 800 kJ/s 3.487 kg/s wnet 229 .39 kJ/kg m a (b) The back work ratio is rbw wC,in wT, out 382 .64 0.625 612 .03 The rate of heat input and the thermal efficiency are a (h3 h2 ) (3.487 kg/s)(1404.7 686.24)kJ/kg 2505 kW Q in m th W net 800 kW 0.319 2505 kW Qin (c) An energy balance on the heat exchanger gives m a (h4 h5 ) m w (hw2 hw1 ) (3.487 kg/s)(792. 62 631 .44 )kJ/kg m w (2792.0 106 .27 )kJ/kg m w 0.2093 kg/s (d) The heat supplied to the water in the heat exchanger (process heat) and the utilization efficiency are Q p m w (hw2 hw1 ) (0.2093 kg/s)(2792 .0 106 .27 )kJ/kg 562 .1 kW u W net Q p 800 562 .1 0.544 2505 kW Q in PROBLEM 9-131 Assumptions 1 The air-standard assumptions are applicable. 2 Kinetic and potential energy changes are negligible. 3 Air is an ideal gas with constant specific heats. Properties The properties of air at 500ºC = 773 K are cp = 1.093 kJ/kg·K, cv = 0.806 kJ/kg·K, R = 0.287 kJ/kg·K, and k = 1.357 (Table A-2b). Analysis (a) For the compressor and the turbine: k 1 Regenerator 6 5 100 kPa 30°C 1 2 700 kPa 260°C Combustion chamber 4 400°C Compress. 871°C 3 Turbine 1.357-1 P k 700 kPa 1.357 T2 s T1 2 303 K 505 .6 K 100 kPa P1 T T (505 .6 303 )K C 2s 1 0.881 T2 T1 (533 303 )K ( k 1) / k P 100 kPa T4 s T3 4 1144 K 685 .6 K P 700 kPa 3 T T4 (1144 T4 )K T 3 0.85 T4 754 .4 K T3 T4 s (1144 685 .6)K (1.357-1)/1.357 (b) The effectiveness of the regenerator is regen T5 T2 (673 533 )K 0.632 T4 T2 (754 .4 533 )K (c) The fuel rate and air-fuel ratio are Q in m f q HV c (m f m a )c p (T3 T5 ) m f (42 ,000 kJ/kg)(0.9 7) (m f 12 .6)(1.093 kJ/kg.K)(1 144 673)K m f 0.1613 kg/s AF Also, m a 12 .6 78.14 m f 0.1613 m m a m f 12 .6 0.1613 12 .76 kg/s f q HV c (0.1613 kg/s)(42,0 00 kJ/kg)(0.9 7) 6570 kW Q in m (d) The net power and the back work ratio are W C,in m a c p (T2 T1 ) (12 .6 kg/s)(1.09 3 kJ/kg.K)(5 33 303 )K 3168 kW WT, out m c p (T3 T4 ) (12 .76 kg/s)(1.09 3 kJ/kg.K)(1 144 754 .4)K 5434 kW Wnet WT, out WC, in 5434 3168 2267 kW W C,in 3168 kW rbw 0.583 WT, out 5434 kW (e) The thermal efficiency is th W net 2267 kW 0.345 6570 kW Q in (f) The second-law efficieny of the cycle is defined as the ratio of actual thermal efficiency to the maximum possible thermal efficiency (Carnot efficiency). The maximum temperature for the cycle can be taken to be the turbine inlet temperature. That is, max 1 and II T1 303 K 1 0.735 T3 1144 K th 0.345 0.469 max 0.735 (g) The exergy efficiency for the compressor is defined as the ratio of stream exergy difference between the inlet and exit of the compressor to the actual power input: T P X C m a h2 h1 T0 ( s 2 s1 ) m a c p T2 T1 T0 c p ln 2 R ln 2 T1 P1 533 700 (12 .6)(1.093 )(533 303 ) (303 ) (1.093 )ln 0.287 ln 2943 kW 303 100 X C 2943 kW II,C 0.929 W C,in 3168 kW The exergy efficiency for the turbine is defined as the ratio of actual turbine power to the stream exergy difference between the inlet and exit of the turbine: T P X T m c p T3 T4 T0 c p ln 3 R ln 3 T4 P4 1144 700 (12 .76 )(1.093 )(1144 754 .4) (303 ) (1.093 )ln 0.287 ln 5834 kW 754.4 100 W T, in 5434 kW II ,T 0.932 X T 5834 kW An energy balance on the regenerator gives m a c p (T5 T2 ) m c p (T4 T6 ) (12 .6)(1.093)(6 73 533 ) (12 .76 )(1.093 )(754 .4 T6 ) T6 616 .2 K The exergy efficiency for the regenerator is defined as the ratio of the exergy increase of the cold fluid to the exergy decrease of the hot fluid: T X regen,hot m c p T4 T6 T0 c p ln 4 0 T6 754.4 (12 .76 )(1.093 )( 754 .4 616 .2) (303 ) (1.093 )ln 0 1073 kW 616.2 T X regen,cold m c p T5 T2 T0 c p ln 5 0 T2 673 (12 .76 )(1.093 )( 673 533 ) (303 ) (1.093 )ln 0 954 .8 kW 533 X regen,cold 954 .8 kW II ,T 0.890 1073 kW X regen,hot The exergy of the combustion gases at the regenerator exit: T X 6 m c p T6 T0 T0 c p ln 6 0 T0 616.2 (12 .76 )(1.093 )( 616 .2 303 ) (303 ) (1.093 )ln 0 1351 kW 303 PROBLEM 9-134 Assumptions 1 The air-standard assumptions are applicable. 2 Kinetic and potential energy changes are negligible. 3 Air is an ideal gas with variable specific heats. P Properties The properties of air are given in Table A-17. q23 3 Analysis (b) We treat air as an ideal gas with variable specific heats, 2 T1 300 K u1 214.07 kJ/kg q12 h1 300.19 kJ/kg 900 K u 2 674.58 kJ/kg h2 932.93 kJ/kg 4 1 300 kPa P2v 2 P1v 1 P 300 K T2 2 T1 T2 T1 P1 100 kPa qout T Pr 4 100 kPa P4 330.9 110 .3 Pr3 P3 300 kPa h4 1036.46 kJ/kg q in q12,in q 23,in u 2 u1 h3 h2 674 .58 214 .07 1395 .97 932 .93 923.55 kJ/kg q out h4 h1 1036 .46 300 .19 736.27 kJ/kg wnet q in q out 923 .55 736 .27 187.28 kJ/kg (c) th wnet 187.28 kJ/kg 20.3% q in 923.55 kJ/kg 3 q23 T3 130 0 K u 3 1022.82 kJ/kg h3 1395.97 kJ/kg, Pr 3 330 .9 v 2 q12 1 4 qout s