Soapmaking as a Green Chemistry Laboratory Experience
advertisement

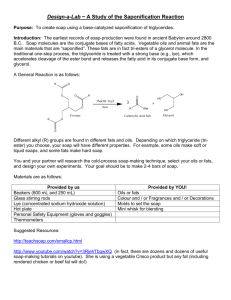
Ma Pearl’s Soap Soapmaking as a Green Chemistry Laboratory Experience Irvin J. Levy and Ronald D. Kay Irv.Levy@gordon.edu Department of Chemistry Gordon College, Wenham, MA 01984 Chemistry is a science of transformation. Recognizing this, the American Chemical Society recently adopted its new vision statement, “Improving people’s lives through the transforming power of chemistry”. In the past decade, another important transformation has also begun: increasing numbers of chemists in both industry and academia have begun to incorporate the principles of green chemistry - chemistry designed to be inherently safer for human health and the environment - into their practice of the discipline. In their seminal work Green Chemistry: Theory and Practice, Paul Anastas and John Warner enumerated “Twelve Principles of Green Chemistry” as guides for the intentional greening of chemical processes.1,2 The teaching laboratory provides an ideal venue for incorporation of these principles, enhancing the opportunity to communicate these goals to our students. The first (and most obvious) of the twelve principles of green chemistry is the admonition to prevent waste. In the early 1990’s, Roger Sheldon suggested the computation of a metric to compare the amount of waste produced in a process to the amount of useful product.3 This easily-computed metric, which Sheldon called the E-factor, is defined by the equation E-factor ≡ mass of waste mass of product The mass of waste is not usually measured in a laboratory. However, since this quantity is equal to the difference of two masses whose values are usually known, namely the mass of all inputs to the process and the mass of the intended product, the E-factor formula can still be found using the formula E-factor = As the Twelve Principles provide guidance for cleaning up the chemical enterprise, the production of soap provides an excellent vehicle to discuss the principles. Numerous laboratory-scale preparations of soap have been published.4-8 The method reported here is closely patterned after Appalachian folkways, reported in The Foxfire Book9, and in several craft books on soapmaking.10-15 This soap-making laboratory is a cooperative project which students can easily complete in a normal lab session (with minor follow up) and which yields enough product that each student can keep a full-sized bar of the soap he/she produces. Soapmaking begins by treating fats or oils with aqueous base to convert their fatty acid esters into long-chain carboxylates. Figure 1 summarizes this reaction, which is called saponification.16 The nature of a soap depends on the fats and oils used in the saponification process as well as on additional materials (e.g., colorants, fragrances, preservatives, exfoliants) added during processing. Traditional folk methods use animal fat such as tallow (rendered beef fat) or lard (rendered pork fat) and very few additives to enhance the aesthetic appeal of the soap. Most craft-oriented books favor the use of vegetable fats and oils for numerous reasons ranging from animal rights to cosmetic properties. Figure 1. Saponification of fats The R groups are typically chains of 12-20 carbon atoms with either no carbon-carbon double bonds (saturated fats) or one to three carbon-carbon double bonds (unsaturated fats). O O R1 R2 O mass of inputs - mass of product mass of product O O O H2O In the best process, one that produces no waste, the E-factor equals zero. While the principle of preventing waste may seem “obvious”, most textbook chemical procedures spectacularly fail to address this principle. In fact, in academic laboratories (where one typically never actually uses the final product for anything) a process arguably produces no useful product and the Efactor becomes infinite! R3 NaOH O O Na R1 R2 Na Na O O R3 OH O HO OH O Additional materials used in the soap-making process described here (but not found in most laboratories) include a food scale with metric graduations, a galvanized 12-quart pail from a hardware store, a large spoon for stirring the soap, a roll of freezer paper, a small cardboard box (9-by-12 inch), a small package of uncooked oatmeal (not instant variety) and several bottles of essential oils. The procedure below employs a mixture of solid Crisco shortening and soybean oil. Use of other fats (as a substitute or in a mixture) requires the amount of sodium hydroxide to be recomputed based on published formulas.17 WARNING! Anyone wishing to create alternate formulations must consult one of the many convenient online calculators18 for guidance in determining the correct amount of sodium hydroxide required. Failure to do so may produce excess lye in the final soap that could make it extremely harsh. Sodium hydroxide as a “traditional” ingredient is available as Red Devil Lye. More involved procedures involve “dripping the lye” by extraction from wood ash.19 We have prepared batches of soap many dozens of times with many different groups, including college organic chemistry students, non-science students, elementary school students, groups of families, and so on. We never used the same formulation twice and our method has never failed to produce useful soap. Accordingly, this method also opens the door to creativity in the hands of the faculty and students who perform the procedure. Caution must be exercised when using the final soap, since it may be rather harsh even when properly prepared and cured. Students may wish to keep their product more for its appearance and fragrance rather than as a cleaning agent; however, as Ma Pearl remarked, “It certainly won’t hurt you. ... If I had’n’a known what lye soap was it’d scare me t’death. You needn’t be scared of that though”.20 As a useful follow-up to the procedure one might ask students to analyze this method in light of the Twelve Principles of Green Chemistry. The method most obviously relates to the following principles: Prevent waste The method given here produces very little waste, except for the small amount of soap that cannot be scraped from the bucket in which it is produced. Also, the freezer paper used to line the mold will end up in the waste stream. Compared to the mass of soap, however, the mass of waste is very small, so the E-factor for this process is close to zero. Maximize atom economy Atom economy measures the number of atoms in the starting materials of a reaction that end up in the final product. For example, an addition reaction has a 100% atom economy because all of the atoms of the starting materials end up in the product. In contrast, in substitution and elimination reactions the atom economy is less than 100% because at least some of the atoms in the starting materials must (by definition) end up in the waste stream. In the soap-making procedure given here, all of the products of the process are combined into the final soap bar. Thus, the atom economy is a perfect 100%. Use renewable feedstocks The oils used in the production of soap are renewable materials. Our original experiences focused on the use of bacon grease for the soap, since this is the fat most commonly used in Appalachian traditions. This is a particularly useful material since it is the waste product of one process (cooking) that has been reassigned as a renewable feedstock in another process (soap production). The production of a practical quantity of soap allows students to explore ways that chemists are “improving chemistry through the transforming power of green principles and practices”.21 ENDNOTES 1. Anastas, Paul T.; Warner, John C. Green Chemistry: Theory and Practice; Oxford University Press: Oxford, 1998. 2. 12 Principles of Green Chemistry (accessed May 15, 2007), http://www.epa.gov/greenchemistry/pubs/principles.ht ml. 3. Sheldon,R. A. “Consider the Environmental Quotient.” CHEMTECH, 1994, 24 (3), 38–47. 4. “Soapmaking, JCE Classroom Activity: #14.” J. Chem. Educ., 1999, 76, 192A. 5. Mabrouk, Suzanne T. “Making Usable, Quality Opaque or Transparent Soap.” J. Chem. Educ., 2005, 82 (10), 1534. 6. Eaton, David C. Laboratory Investigations in Organic Chemistry; McGraw-Hill, Inc.: New York, NY, 1989, 537-538. 7. Campbell, Jr., Bruce N; Ali, Monica McCarthy Organic Chemistry Experiments, Microscale & Semi-Microscale; Brooks/Cole Publishing Company: Pacific Grove, CA, 1994, 420-421. 8. Svoronos, Paris; Sarlo, Edward Organic Chemistry Laboratory Manual; Wm. C. Brown Publishers: Dubuque, IA, 1994, 287. 9. Wigginton, Eliot, ed. The Foxfire Book; Anchor Books: Garden City, NJ, 1972. 10. Cavitch, Susan Miller The Soapmaker’s Companion; Storey Publishing: Pownal, VT, 1997. 11. Makela, Casey Milk-Based Soaps; Storey Publishing: Pownal, VT, 1997. 12. Maine, Sandy The Soap Book; Interweave Press: Loveland, CO, 1995. 13. Bramson, Ann Soap; Workman Publishing: New York, NY, 1975. 14. Mohr, Merilyn The Art of Soapmaking; Firefly Books: Buffalo, NY, 1979. 15. Cavitch, Susan Miller The Natural Soap Book; Storey Publishing: Pownal, VT, 1995. 16. Solomons, T.W. Graham; Fryhle, Craig B. Fundamentals of Organic Chemistry, 8th edition, John Wiley and Sons: New York, NY, 2004, 1135-1137. 17. The Soapmaker’s Companion, 247. 18. MMS Lye Calculator (accessed May 15, 2007), http://www.the-sage.com/calcs/lyecalc2.php. 19. The Foxfire Book, 155-157. 20. The Foxfire Book, 154. 21. Kay, Ronald D.; Levy, Irvin J. “Propagation of the principles of green chemistry via ‘memetic catalysis’: Student motivated endeavors advancing green organic literacy.” Presented at the 231st ACS National Meeting, 10-14 September 2006, Abstract CHED 0472. 22. We found that waxed paper does not work well. Heavy plastic-coated freezer paper works very well. 23. Careful measurement of the NaOH is necessary in this step in order to ensure that an excess is not present in the final soap. The amount of grease used in the procedure should actually require 272 g NaOH for total saponification. This amount has been reduced to 245 g (90% of 272 g; referred to as a “10% discount” in soapmaking books). Discounts of 5 to 20% are common. 24. Available at craft stores or health food (or other retail) stores which stock aromatherapy supplies. For a more complete selection of essential oils, see lists of suppliers in craft soap books. 25. Some crayons will cause the soap to be speckled with color; others will impart a homogenous color throughout the soap. We have found that one green Crayola crayon will give the batch of soap an attractive light green color, quite appropriate for a green chemistry laboratory! 26. When using other fats and oils the initial saponification and setting may require more than 24 hours. Do not attempt to unmold and cut the soap if it is not firm to the touch. With some oils this may take as long as 2 weeks. We have generally had best success with mixtures of 50-100% solid fats mixed with liquid oils. Ma Pearl’s Soap CAUTION! Preparation and use of sodium hydroxide solution is hazardous. Use goggles, gloves and fume hood. Sodium hydroxide becomes very hot when dissolving in water. Use caution to avoid splashing the caustic solution on skin or in eyes. Do not breath vapors from the solution. PROCEDURE Line a small cardboard box with freezer paper22 so the soap can be poured into the box as a mold. Take care to avoid large creases in the paper and gaps at the corners that would allow the liquid to spill through. Tape edges of the paper at the top to hold them in place. Dissolve 245 g NaOH in about 500 mL of water.23 Stir constantly to prevent a solid mass of sodium hydroxide from clumping on the bottom of the container. The solution will become quite hot. Cool the sodium hydroxide solution in a water bath until the temperature is about 35-40°C. Transfer 1.0 kg of solid Crisco shortening and 1.0 kg liquid soybean oil into a galvanized 12-quart pail, using a food scale to measure the amount of grease added. Warm on a hotplate until the solid fat melts. Stir the fat while warming. After a liquid is obtained, cool in a bucket of water to a temperature of 35-40°C. Continually stir while cooling to avoid formation of solids on the inner surface of the metal pail. Note that in a cooperative laboratory setting, the first three tasks (preparation of the lined box, preparation of the sodium hydroxide solution, and preparation of the fats) can be completed by three teams of students working in parallel. Once the sodium hydroxide solution and melted fats are both at the proper temperatures, carefully add the sodium hydroxide solution to the fat with stirring. Stir as vigorously as possible without splashing. While stirring it is important to occasionally scrape the liquid from the sides of the pail down into the mixture. Stirring con- tinues until the mixture has become thick enough that one can faintly trace lines onto the surface of the liquid in the pail by drizzling a little liquid from the spoon onto the surface. In our hands this required roughly 90 minutes; actual time needed may vary. Be sure to stir to this “trace stage,” but don’t continue beyond that point or it will be difficult to pour the soap into its mold. It is helpful for students to take five-minute turns at stirring. While stirring progresses, others can prepare additional ingredients. We found that two handfuls (traditional measuring units) of uncooked oatmeal give the soap an interesting appearance. Gently mill the oatmeal by hand until it has a medium grained texture. As soon as the soap has reached the trace stage, fold the oatmeal into the mixture, stirring well. If desired, add 15-30 mL of essential oils24 in a slow, steady stream with continuous stirring. Color can be imparted to the final soap by the addition of small amounts of spices (e.g. cinammon, cocoa powder) or by dissolving a crayon in 10-20 mL warm soybean oil.25 Blend all of the materials well. Pour the soap into the mold and cover the top with towels, plywood or any other insulating material. Let the soap rest in the mold for at least 24 hours. During this time the saponification will continue. Wait until the large slab of soap reaches the consistency of soft clay26 before continuing (usually 2-7 days). Remove the soap from the box by gently lifting the paper liner out. The soap is conveniently sliced into bars. Alternately, thin slices of the slab can be cut off and rolled into soap balls. Creative individuals might wish to mold the clay-like soap into interesting shapes at this point. Avoid contact with the skin since the raw soap may still be quite alkaline at this stage. Form all of the soap bars into their final shapes at this time. Within a few days some surfaces of the soap may possess a powdery residue of sodium carbonate or unreacted sodium hydroxide. Using a sharp knife, slice this thin layer from the soap. Allow the soap to cure by wrapping in paper towels and air-drying for 4-6 weeks before considering use. The yield is approximately twenty-four 2-by-3-inch bars of soap, depending on thickness. CAUTION: The final cured soap may still be relatively harsh and care should be taken in using the final product, especially in the case of individuals with sensitive skin. The final product may contain unreacted sodium hydroxide, a severe irritant. Take care to avoid contact with the eyes. It is recommended that the final product be used only for decorative purposes, though when properly prepared as described, the author has obtained a product suitable for use as a hand soap.