Twinrix Policy - Columbus County
advertisement

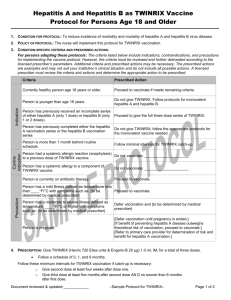
COLUMBUS COUNTY HEALTH DEPARTMENT TWINRIX POLICY Policy Title: Twinrix Policy Program Area: All Clinical Areas Policy Identifier: (optional) Effective Date: Approval Date: Revision Date(s): Approved by: Kim Smith RN, BSN, MSHCA, Health Director Approved by: Hilda Memory RN, BS, MSHA, Director of Nursing 1/2/2004 8/18/08, 9/21/2010 Purpose: The purpose of this policy is related to the availability of Twinrix vaccine to local health departments effective January 2, 2004. Twinrix, manufactured by GlaxoSmith Kline, is a hepatitis A and hepatitis B combination vaccine for use in persons > 18 years of age and older. The Center of Disease Control and Prevention in Atlanta recommend this vaccine for certain high risk populations. Definitions: Columbus County Health Department will make available State Supplied Twinrix Vaccine Administration and administer in accordance to eligibility criteria. Responsibilities: Public Health Nurse(s) Procedures: It is administered in a three dose series, by the same schedule as single-antigen hepatitis B vaccine for adults. o The first dose is administered o The second dose is administered one month later. o The third dose is administered 6 months after the first dose or 5 months after the second dose. Reformatted 2010 Page 1 of 3 COLUMBUS COUNTY HEALTH DEPARTMENT TWINRIX POLICY The state supplied Twinrix is packaged as five single dose safety Tip Lock syringes without needles. You will need to purchase needles for use with this product. Eligibility: Twinrix vaccine is available only for local health departments. Individuals eligible for state supplied Twinrix vaccine are defined as > 18 years of age who present to the local health department for any reason with any of the following risk factors: o Men who have sex with men, o IV drug users, o Persons with multiple sex partners, o Any person who has been incarcerated, o Persons who are hepatitis C or HIV positive, o Persons with chronic liver disease, including persons with chronic HBV/HCV infections who have evidence of chronic liver disease, or o Persons seeking treatment for STD. Contraindications: Hypersensitivity to any component of the vaccine, including yeast and neomycin, is a contraindication. This vaccine is contraindicated in patients with previous hypersensitivity to TWINRIX or monovalent hepatitis A or hepatitis B vaccine. Precautions: A moderate or severe acute illness is sufficient reason to postpone vaccination. TWINRIX should be administered with caution to people on anticoagulants, and those with thrombocytopenia or a bleeding disorder since bleeding may occur following intramuscular administration. As with any parenteral vaccine, epinephrine injection (1:1000) and other appropriate agents used for the control of immediate allergic reactions must be immediately available should an acute anaphylactic reaction occur? Reformatted 2010 Page 2 of 3 COLUMBUS COUNTY HEALTH DEPARTMENT TWINRIX POLICY Adverse Reactions: See package insert http://www.twinrix.com/risk-side-effects.html Patient Teaching: Patients should be informed of the benefits and risk of immunization with TWINRIX, and of the importance of completing the immunization series. For further information refer to the following website: http://www.cdc.gov/vaccines/pubs/ACIP-list.htm Laws and Rules: Reference(s): CDC.gov GlaxoSmith Kline Reformatted 2010 Page 3 of 3