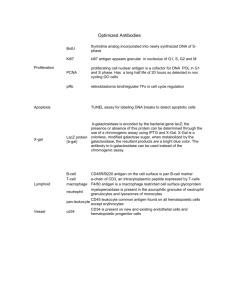
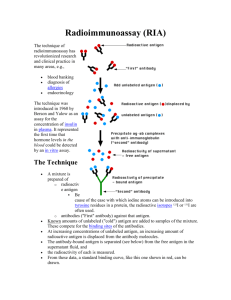
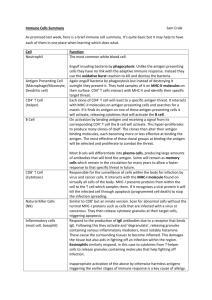
Antigen Submission Form
advertisement

Raw Material(s) for Hybridoma Development Information Form Instructions 1. Please complete and mail a hard copy of this form together with your antigen and all additional information to Antibody Services, GenScript Corp., 860 Centennial Ave, Piscataway, NJ 08854, USA 2. * Mandatory information Customer Information Account number: Order ID or Quotation ID: A. B. C. D. E. F. Immunization Antigen Information Positive screening/purification Antigen Information Negative screening/purification Antigen Information Immunization Cell Line Information Negative Control Cell Line Information General amount and COA requirements for materials to be submitted A. Immunization Antigen Information Type of antigen*: Soluble Protein Membrane Protein Bacteria Cell line Antibody Peptide Gene-to-Protein-to-Antibody in GenScript Other: Carbohydrate Lipid Virus If cell line is selected, please fill target protein information in this form and fill Cell line information table D. NOTE: If the antigen is recombinant protein, please describe which fusion tag was used. 0.5-1 mg tag alone or generic protein with the same tag is requested for monoclonal counter-screening or 3 mg for polyclonal antibody cross-absorption. Otherwise, tag cleaved recombinant protein should be provided. Is your antigen toxic or harmful to humans/animals? Yes No If Yes, please specify: Name of Immunization Antigen*: Species of Immunization Antigen*: Human Mouse Rat Yeast E.coli Does your antigen have known homologs in other species? Yes No If YES, please specify: Mouse Rat Other animal: Identities rate: Yes Is the sequence or molecular structure of the antigen available? Other: No If YES, please include the sequence information: Molecular Weight*: If the antigen MW less than 8 kDa, GenScript will request antigen to be conjugated to carrier protein (KLH, BSA, OVA, etc.) to enhance immune response. Format (e.g. liquid, lyophilized, gel, etc.) : How to reconstitute if it’s lyophilized? Tag or conjugate*: Buffer components: Storage/shipping temperature: Number of vials: Volume per vial and concentration: Precautions: Certificate of Analysis of your material: Please attach your COA data of your customer antigen. (ex. SDS-PAGE/HPLC/MS for purity/MW/quantity, etc.) B. Positive screening/purification Antigen Information Antigen P1. Type of antigen Antigen P2. Antigen P3. Antigen name Sequence or molecular structure* MW Format Tag or conjugate* Buffer components Storage/shipping temperature Number of vials Concentration COA How to use (Recommended usage) Precautions C. Negative screening/purification Antigen Information Antigen N1. Antigen N2. Antigen N3. Type of antigen Antigen name Sequence or molecular structure* MW Format Tag or conjugate* Buffer components Storage/shipping temperature Number of vials Concentration COA How to use (Recommended usage) Precautions D. Immunization Cell Line Information Name of Cell line*: Name of Cell line on Vial Label*: Format: Frozen (>2 vials of frozen cells, >106 cells/vial) Number of vials: Growth Conditions and Media Requirements: Mycoplasma Test Result: Positive Negative Unknown Number of cells/vial: Note: Our default culture medium is DMEM with 10%FBS and IMDM, RPMi can be selected as well. If you require the special culture medium, an additional fee will be charged. Target protein information*: Please fill Table A Certificate of Analysis of your antigen: The target protein expression level should be >50,000 copies/cell or more than one log shift on flow cytometry assay. Precautions: E. Negative Control Cell Line Information Name of Cell line*: Frozen (>2 vials of frozen cells, >106 cells/vial) Number of vials: Format: Name of Cell line on Vial Label*: Mycoplasma Test Result: Positive Number of cells/vial: Negative Unknown Cell density/volume: Growth Conditions and Media Requirements: Note: Our default culture medium is DMEM with 10%FBS and IMDM, RPMi can be selected as well. If you require the special culture medium, an additional fee will be charged. Target protein information: Precautions: F. General amount and COA requirements for materials to be submitted Screen materials Fusion tag The purified protein Cell line Bacteria/Virus Activated protein/antibody Plasmid Total quantity > 0.5 mg > 0.5 mg > 106 cells > 108 clones > 0.5 mg >50µg WB Test materials The purified protein The transfected cell Cell lysate Tissue lysate Total quantity 10µg >1 mg total protein >1 mg total protein >1 mg total protein Total concentration >0.4 mg/ml >0.4 mg/ml >108 /ml >0.4 mg/ml Total concentration >0.5 mg/ml >2 mg/ml >2 mg/ml >2 mg/ml COA (Not limited) WB SDS-PAGE FC/WB/ICC Inactivation Activity assay Sequencing/vector COA (Not limited) SDS-PAGE WB/FC Project Information Is this project for grant application purposes? When will the project start? Immediately Within one month Yes No Within 3 months Half a year or more