Biocoagulation as part of a new technology for separation of fine
advertisement

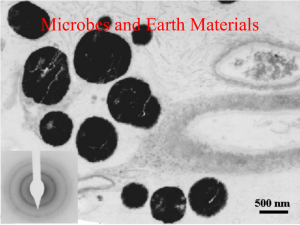
Biocoagulation as part of a new technology for separation of fine particles Jana Pinka Technical University Berlin, Mechanical Process Engineering & Solids Processing Microbes can enrich or degrade particles Figure 1: Process design due to different charges of particles and microbes. This fact is exploited in microLoading by Biocoagulation biological separation processes like bioaccumulation, bioprecipitation and bioSorting by sorption. Such processes are used to ree.g. Flotation cover metals, either valuable or nuisance, from bleeds and effluents from bioleachSeparation by e.g. Washing ing operations and other hydrometallurgical treatments. Still, the transport of partiSorting products Microorganism cles by microbes is not well-investigated [1]. Biocoagulation new technology for making extremely fine-grained particles collectible, and differs from well-known processes like biosorption [2], bioaccumulation and biotransformation: In biocoagulation the particles are very small and solid. For the other processes, the minerals dissolve and the ions are adsorbed or accumulated. Mikroorganismus Sortierprodukte The investigations follow three major directions (figure 1): Firstly, the different behaviour of particles, minerals and microbes, in electrolytes is used. Between the electrolyte and the particle surface, a transition of charge carriers (electrons, ions) often takes place. Thereby the charge carriers can migrate into the liquid phase or vice versa - adsorb to the solid phase. Because of the enrichment of charge carriers, an electrochemical bilayer originates consisting of a relatively fixed layer (Stern Layer) and a more diffuse layer (Gouy-Chapman-Layer). The Electrokinetic potential of the shearing layer in direction Potential to the neutral solution is called zetapotential. Different zeta-potentials at the surfaces of minerals and microbes are connecting the minerals and microbes into clusters. Measurements of the zeta-potential were performed to find the best conditions for coagulation of the model minerals Galena and Sphalerite and the yeasts Saccharomyces cerevisiae and Yarrowia lipolytica. Distance r Saccharomyces cerevisae Figure 2: Potentials of the electrochemical bilayer. The charge carriers may be the metal ions one wants to collect Sphalerite The yeasts were cultivated, gathered and provided for coagulation tests. Microscopic studies demonstrate the adhesion of the sulphidic minerals onto the yeasts’ surface. [1] Bowen et al. (2001) J Colloid Interface Sci. 1, 237, 54-61 [2] Strouhal et.al. (2003) Bioelectrochemistry 60, 29-36 15.02.2016 Yarrowia lipolytica Figure 3: Microscopic pictures showing different yeasts adhering to the model mineral Sphalerite