How can we characterize the “absorbent capacity”
Introduction
How can we characterize the “absorption capacity” of a superabsorbent polymer?
Superabsorbent polymers are substances that can absorb and retain extremely large amounts of a liquid relative to their own mass. In particular, a class of polymers called crosslinked polyacrylates are excellent water absorbing products. They are extensively used in personal disposable hygiene products such as baby diapers and sanitary napkins. Other applications include horticultural water retention, control of spill and waste aqueous fluids, humidity control, blocking water penetration in underground power or communications cables, water-blocking tape, artificial snow for motion pictures, and even as fire retardants.
Sodium polyacrylate is one of the most common superabsorbent polymers.
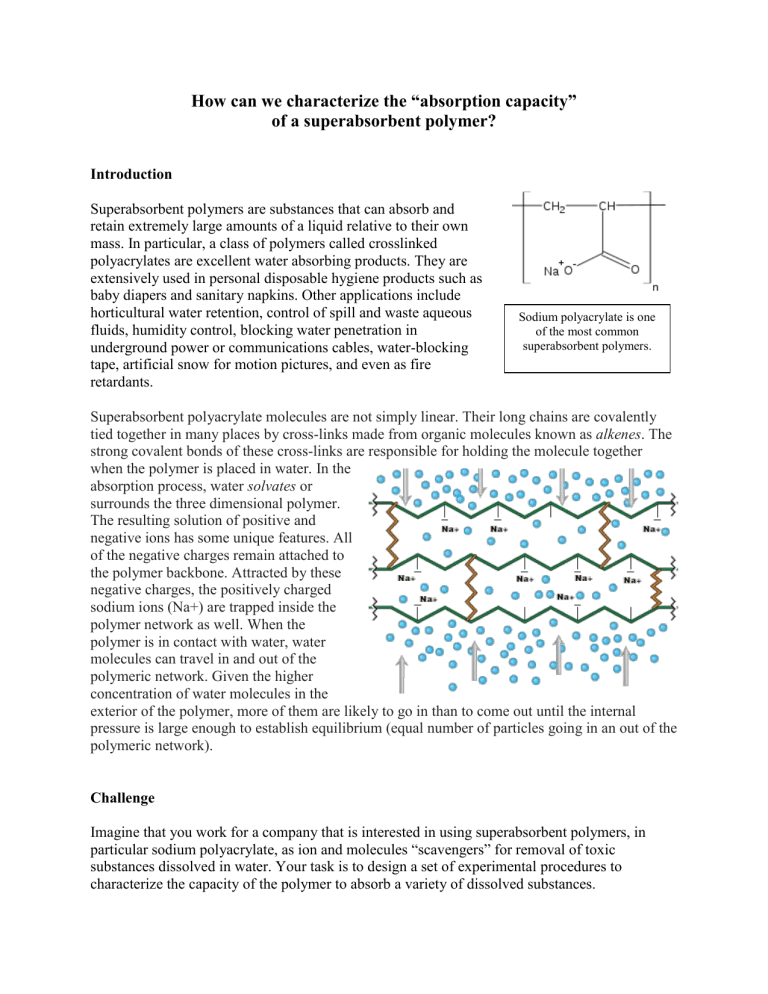
Superabsorbent polyacrylate molecules are not simply linear. Their long chains are covalently tied together in many places by cross-links made from organic molecules known as alkenes . The strong covalent bonds of these cross-links are responsible for holding the molecule together when the polymer is placed in water. In the absorption process, water solvates or surrounds the three dimensional polymer.
The resulting solution of positive and negative ions has some unique features. All of the negative charges remain attached to the polymer backbone. Attracted by these negative charges, the positively charged sodium ions (Na+) are trapped inside the polymer network as well. When the polymer is in contact with water, water molecules can travel in and out of the polymeric network. Given the higher concentration of water molecules in the exterior of the polymer, more of them are likely to go in than to come out until the internal pressure is large enough to establish equilibrium (equal number of particles going in an out of the polymeric network).
Challenge
Imagine that you work for a company that is interested in using superabsorbent polymers, in particular sodium polyacrylate, as ion and molecules “scavengers” for removal of toxic substances dissolved in water. Your task is to design a set of experimental procedures to characterize the capacity of the polymer to absorb a variety of dissolved substances.
Your Tasks
Task 1
Goal: You need to design and implement an experimental procedure to quantitatively determine how the water absorption capacity of sodium polyacrylate varies as a function of sodium chloride concentration . Absorption capacity is defined as the maximum amount of liquid that the polymer can hold without spilling. This test is important because many natural aqueous systems in which the polymer will be used contain different concentrations of salts dissolved in them (e.g., fresh and sea water), and this may affect the efficiency of your product.
Expected outcomes: You should be able to generate a graph that shows how the volume of water absorbed per gram of polymer changes with NaCl concentration (the graph should include at least four different salt concentrations, one of them corresponding to tap water).
Resources: 1.0 g of sodium polyacrylate (don’t use more than 0.25 g per test), 10.0 g/L NaCl solution
(you will need to prepare any necessary diluted solutions), absorbent paper, plastic stirring rod, volumetric glassware (we recommend you use 250 mL beakers), digital scale.
Task 2
Goal: You need to design an experiment to quantify the mass of copper (in Cu
2+
form dissolved in water) that sodium polyacrylate can absorb per gram of this polymer as a function of time . This will allow you to explore the efficiency of this polymer to remove toxic metal ions from the environment.
Expected outcomes: You should be able to generate a graph that shows the mass of Cu
2+
absorbed per gram of polymer as a function of time (in minutes). This data should allow you to estimate the maximum mass of copper absorbed per gram of polymer. Your results should be reproducible.
Resources: 0.25 g of sodium polyacrylate, tea bag, 100 mL of 0.05 M Cu(NO
3
)
2
solution, absorption spectrometer, stopwatch, magnetic stirrer and stir bar, volumetric glassware.
Note: Tea bags can be used to put the polymer in contact with the solution without getting dispersed throughout the fluid.
Task 3
Goal: You should pose a research question to investigate in the lab, and design and implement and experiment to answer it.
The answer to this question should allow you to expand your understanding of the absorption capacity of sodium polyacrylate and its potential use as substance scavenger.
Expected outcomes: You should generate the answer to the research question that you pose. Your answer should include both qualitative observations and quantitative data.
Resources: 1.0 g of sodium polyacrylate, tea bags, 10.0 g/L NaCl solution, 0.05 M Cu(NO
3
)
2 solution, 0.05 M Co(NO
3
)
2
solution, 0.05 M Ni(NO
3
)
2
solution, solid CaCl
2
, 6.96 x 10
-6
M
FD&C Dye Blue #1 solution, 4.03 x 10
-5
M FD&C Dye Red #40 solution, absorption spectrometer, stopwatch, magnetic stirrer and stir bar, absorbent paper, volumetric glassware.
THINKS TO REMEMBER:
1.
Complete the IBIS Lab/Disc quiz associated with the two-week experiment “How can we use chemical reactions to identify substances?” before Sunday, October 31 at 11 PM.
2.
Complete and bring a hard copy of your lab report for the two-week experiment “How can we use chemical reactions to identify substances?”
3.
Bring your laptop to the lab.
THINKS TO DO BEFORE COMING TO LAB:
1.
Carefully analyze each of the three major tasks associated with the experiment “How can we characterize the absorption capacity of a superabsorbent polymer?” (The corresponding handout is posted in the Content section of D2L). It is important that you complete the following tasks before coming to lab. a) Task 1 : Design a procedure to measure the amount of water that sodium polyacrylate can absorb as a function of NaCl concentration. Sodium polyacrylate is a solid powder that will absorb water and form a gel, how can you reliably determine when the polymer stops absorbing water? b) Task 2 : Design a procedure to quantify the mass of copper (in Cu 2+ form dissolved in water) that is absorbed by the polymer as a function of time. Copper solutions are colored, how could you use absorption spectroscopy to follow changes in the concentration of Cu
2+
in an aqueous solution as a function of time? Review the section on absorption spectroscopy in your lab manual, and your own lab reports form past experiments, to help you design this experiment. c) Task 3 : Come up with a research question that can be investigated and answered in the lab given the listed resources. Your question should help you gather more information about the absorption properties of sodium polyacrylate. Design an experimental procedure to answer your question.
IMPORTANT NOTE : You are expected to type down (in one to two pages total) three reasonable procedures to complete each of the tasks of this experiment, together with the research question you plan to answer during Task 3. Your TA will collect this work during the lab and use it as part of the lab evaluation. Given that you will not be asked to write a lab report for this experiment, your pre-lab work will have a heavier weight on your lab grade. Thus, it is important that you complete this assignment and do a good job with it.










