Geologic Time II
advertisement

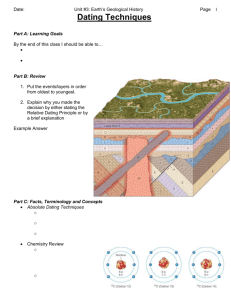
Geologic Time II Atomic Chemistry Radiometric Dating Radiometric Dating • Makes use of the fact that some isotopes spontaneously decay – Change involves a parent and a daughter element – Always involves changes in the nucleus • Nuclear reactions—changes in the nucleus – – – – – Alpha Emission Beta Decay Electron Capture Positron Emission Gamma Ray Emission Decay Processes • Alpha Emission () – Alpha particle emitted (2 neutrons & 2 protons—nucleus of a helium atom) – Atomic mass decreases by 4 – Atomic number decreased by 2 – Important in decay scheme of Uranium Decay Process • Beta Decay ( ) - – Beta particle (electron) emitted • Neutron decays to a proton and electron – Atomic mass is unchanged – Atomic number increases by 1 – 14C decays to 14N Decay Process • Electron Capture – Electron is captured and combines with a proton to form a neutron – Atomic number decreases – Atomic mass remains the same – 40K decays to 40Ar Decay Process • Positron Emission (+) – Positron is a particle with the same mass as an electron (nearly zero), but with a positive charge – A proton basically changes into a neutron – A proton’s charge is lost – A neutron is gained – The atomic number decreases and the atomic mass remains constant Decay Process • Gamma Ray Emission () – Short-wavelength, high-energy electromagnetic rays – Affects neither atomic number nor the atomic mass – Happens when one of the other decay mechanisms occurs and the newer isotope shifts to a lower, more stable, energy state Principles • Alpha emission, Beta decay, and electron capture are the most important decay mechanisms for radiometric dating • Parent isotopes decay to daughter isotopes, but the total number of atoms remains the same • Decay occurs at a constant rate – Expressed in terms of half life—the amount of time it takes for half of the isotopes to decay – Collisions enhance decay, so decay will depend on how many atoms there are to start with Principles • To make dating work you need – Original amount of material, both parent and daughter – Decay rate – Present amount of material, both parent and daughter • Decay rate is known, and present amount of material can be measured • Most radiometric dating techniques generally rely on some special circumstance to estimate the original amount of material Fission Track Dating • Some minerals, such as apatite, incorporate uranium into the crystal structure • As the uranium decays it damages the crystal and leaves little tracks – Tracks become visible when crystal is etched with hydrofluoric acid – Need to be viewed under a microscope Fission Track Dating • The number of tracks is proportional to how much uranium has decayed • Since we can measure the amount of uranium currently in the crystal and we can count the tracks to determine how much has decayed, we determine how much was originally present • From those data, it is possible to calculate an age for the sample Fission Tracks Fission Track Dating • Heating anneals the crystal and wipes out the tracks – This means there were no tracks until the crystal had cooled – Reheating can mess up the date • Most useful for rocks between 40,000 and 1.5 million years old • Very often used to date age of uplift, that is when the uplift brought the rock close enough to the surface to cool off Carbon-14 Dating • Three isotopes of carbon – – – 12C = 98.892% = 1.108% 14C = 1.07 x 10-10% 13C • Carbon-14 is produced in the atmosphere by bombardment of nitrogen-14 • If cosmic flux is constant, a steady state will be reached and the 14 C/12C ratio will be constant Carbon-14 Dating • Plants take up carbon (independent of the isotope) • When the plant dies, it stops taking up carbon, and carbon-14 decays – The ratio of 14C/12C will decrease – We can measure the 14C/12C ratio in dead plant material – It is then a simple matter to calculate how long the ratio has been decreasing by comparing the measured ratio with the constant value Carbon-14 Dating • Problem – Cosmic flux has not been constant, and therefore, the constant – Calibrate against tree ring data, and correct • This has been done for the last 6000 or 7000 years 14C/12C ratio is not • For ages greater than about 7000 years, the changes in cosmic flux average out, and the error is not significant • Constraints – Must have a source of carbon – Short half life (5730 years) means the method is only useful for stuff less than 70,000 years – It’s really reliable only for stuff < 7000 years Potassium-Argon Dating • Three isotopes of potassium, of which 40K is the least abundant • Three isotopes of argon, of which 40Ar is most abundant • 40K decays to both 40Ca and 40Ar – 89% to Ca, 11% to Ar – 40Ca is so abundant, that branch is useless • Measure amount of 40K and 40Ar currently in rock Potassium-Argon Dating • Since argon is a gas, there was none originally • Therefore, all argon in the rock is radiogenic • Simply calculate the length of time needed to produce that much argon given the amount of potassium currently present Potassium-Argon Dating • Problems – Argon can escape giving too young an age – Argon is likely to escape until the rock cools below 200°C – Excess argon trapped initially giving an age that is too old – Care is necessary • Most useful in rocks with lots of potassium, that cooled quickly, and that are pretty old (half life is 1.3 billion years) Uranium-Lead • Uranium has three important isotopes – 238U which decays to 206Pb (99.28%) – 235U which decays to 207Pb (0.71%) – 234U which is not relevant to us (0.0058%) • Lead has 4 important isotopes – 204Pb which is essentially constant (1.37%) – 206Pb produced by decay of 238U (26.26%) – 207Pb produced by decay of 235U (20.82%) – 208Pb produced by the decay of 232Th (51.55%) Uranium-Lead Decay Scheme Uranium-Lead • The ratio of 238U/235U changes at a known rate, and can be calculated for any time • Concordia plot – – – – – Plot 206Pb/238U on vertical axis Plot 207Pb/235U on horizontal axis Calculated curve based on known 238U/235U ratio gives concordant ages Calibrate curve Plot your samples • Plot on curve gives age • Plot on line below curve gives a lead-loss event