Freezing Down HT22 Cells
advertisement

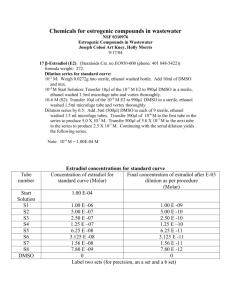
KT 12/14/05 Freezing Down HT22 Cells 1. Cells must be at least 50% confluent and have more than one flask of each. (one flask usually results in about 1mL of frozen cells) 2. Suck off media and add 5 ml trypsin 3. Let sit for 3-5minutes until cells are floating 4. Add 5ml of media to deactivate trypsin 5. Spin at 3000RPM for 10min 6. Suck off media 7. Add 5ml of new media to cell pellet mix gently 8. Add 400uL of trypan blue to eppitube 9. Take 100uL of cell mixture and add to eppitube too 10. Cell count = (#/quadrants) x 5 x 10,000 = total cells 11. Divide this by 1 x 10^6 or 2 x 10^6 that equals #mLs of FBS/DMSO to add to cells 12. Centrifuge again at 3000RPM for 10min 13. Suck off media 14. Add appropriate amount of FBS/DMSO, 10%DMSO 90%FBS 15. Mix pellet in solution 16. Add cell solution to a Nunclon CryoTube Vial 17. Label Date, Cell type, Split number, and what cell is frozen in, i.e. DMSO. 18. Freeze in -80°C for approx. 1-2 hrs 19. Freeze in liquid nitrogen 20. Make sure and log location (rack and box #) of tubes in the folder on the shelf in the Levitt scope room.