GCSE Chemistry – Unit 1 Factsheets
advertisement

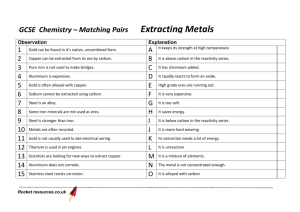
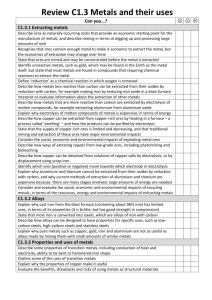
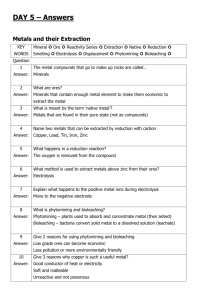
GCSE Chemistry – Unit 1 Factsheets 1.2 How do rocks provide building metals & how are metals used? a) Ores Ores contain enough metal to make it economical to extract the metal and this changes over time, eg aluminium is obtained from bauxite as the cost of getting hold of the bauxite and using it to produce pure aluminium is low enough to make a profit. If costs changed, we might be forced to obtain aluminium by another means or use less aluminium. Chemistry and finance are often connected. b) How the reactivity series relates to obtaining pure metals Unreactive metals such as gold are found in the earth as the metal itself. Most metals are found as compounds that require chemical reactions to extract the metal. Metals that are less reactive than carbon can be extracted from their oxides by reduction with carbon (via carbon monoxide), eg Iron in the Blast Furnace Iron oxide + Carbon Iron + Carbon dioxide; Fe2O3(l) + 3CO(g) 2Fe(l) + 3CO2(g) monoxide (Metals which are more reactive than carbon are extracted using electrolysis) c) Iron & Steel Iron from the Blast Furnace contains about 96% iron. The impurities make it brittle and so it has limited uses. Removing Defn: Alloy – mixture of a metal with all of the impurities would produce other metals or with a substance pure iron. Pure iron has a regular such as carbon in order to change the arrangement of atoms, with layers properties of the metal. that can slide over each other, and so is soft and easily shaped, but too soft for many uses. Most iron is converted into steels. Steels are alloys since they are mixtures of iron with carbon and other metals. The different sized atoms added distort the layers in the structure of the pure metal, making it more difficult for them to slide over each other, and so alloys are harder. Alloys can be designed to have properties for specific uses. Low carbon steels are Properties & uses of steels: easily shaped, high carbon steels Low carbon – easily shaped, ie car bodies are hard, and stainless steels are High carbon – hard, ie construction work resistant to corrosion. Stainless – resists corrosion, ie cutlery d) Other alloys Many metals in everyday use are alloys. Pure copper, gold, and aluminium are too soft for many uses and so are mixed with small amounts of similar metals to make them harder for their everyday use. Brass & bronze are alloys of copper. White gold is an alloy of gold. Alloys containing aluminium are used in planes. e) Smart alloys Smart alloys (also called shape memory alloys) can return to their original shape after being deformed. They are used in frames for glasses and teeth braces. f) Transition metals The elements in the central block of the periodic table are known as transition metals. Like other metals they are good conductors of heat and electricity and can be bent or hammered into shape. They are useful as structural materials and for making things that must allow heat or electricity to pass through them easily. g) Copper Copper has properties that make it useful for electrical wiring and plumbing. Copper can be extracted by electrolysis of solutions containing copper compounds. Ores rich in copper are in short supply. Therefore, new ways of extracting copper from low-grade ores are being researched to limit the environmental impact of traditional mining. h) Aluminium & Titanium Aluminium and titanium are useful as their densities are low and they resist corrosion. They cannot be extracted from their oxides by reduction with carbon as they are more reactive than carbon. Current methods of extraction are expensive because, there are many stages in the processes and much energy is needed. i) Recycling Recycling metals is important because extracting them uses limited resources and is expensive in terms of energy and effects on the environment. Extraction & uses of Aluminium & Titanium Aluminium is extracted from bauxite ore using electrolysis. Aluminium is used to make planes; pots & pans; aluminium cans; cooking foil; etc Titanium is extracted from rutile ore by displacement reactions involving a reactive metal such as sodium or magnesium. Titanium is used for space vehicles; hip joints; sports equipment; planes; etc Why recycle aluminium? Aluminium ores could run out Extraction of aluminium uses lots of electricity and so is very costly Production of the greenhouse gas carbon dioxide during the process Loss of landscape due to mining, processing & transporting bauxite ore. Loss of landscape for electrolysis plant Noise pollution Pollution involved in energy generation Avoids need to dump old aluminium if it wasn’t recycled. Typical AQA style Examination Questions 1. The table below gives information about some metals Name of the metal Cost of one tonne of metal (£) Aluminium Platinum Iron Gold 883 16 720 000 216 8 236 800 % of metal in earths crust 8.2 0.0000001 4.1 0.0000001 a) Use the information in the table to suggest why gold and platinum are very expensive metals ………………………………………………………………………………………………………………………………………………………………. ………………………………………………………………………………………………………………………………………………………………. b) Aluminium and iron are made by reduction of their ores Name the element that is removed from their ores when they are reduced ………………. Suggest a metal that would reduce aluminium ore ……………………………………………………………… c) Aluminium is made by the reduction of molten aluminium ore, using a very large amount of electricity. How is iron ore reduced in a blast furnace to make iron? ………………………………………………………………………………………………………………………………………………………………. ………………………………………………………………………………………………………………………………………………………………. ………………………………………………………………………………………………………………………………………………………………. ………………………………………………………………………………………………………………………………………………………………. Suggest why aluminium is more expensive than iron ………………………………………………………………………………………………………………………………………………………………. ………………………………………………………………………………………………………………………………………………………………. 2. Discuss whether the re-cycling of fizzy drink cans is worthwhile. Give both sides of the argument ………………………………………………………………………………………………………………………………………………………………. ………………………………………………………………………………………………………………………………………………………………. ………………………………………………………………………………………………………………………………………………………………. ………………………………………………………………………………………………………………………………………………………………. ………………………………………………………………………………………………………………………………………………………………. ………………………………………………………………………………………………………………………………………………………………. ………………………………………………………………………………………………………………………………………………………………. ………………………………………………………………………………………………………………………………………………………………. 3. Discuss the differences that exist between group 1 metals and the transition metals. Make reference to specific elements in your argument ………………………………………………………………………………………………………………………………………………………………. ………………………………………………………………………………………………………………………………………………………………. ………………………………………………………………………………………………………………………………………………………………. ………………………………………………………………………………………………………………………………………………………………. ………………………………………………………………………………………………………………………………………………………………. ………………………………………………………………………………………………………………………………………………………………. ………………………………………………………………………………………………………………………………………………………………. ………………………………………………………………………………………………………………………………………………………………. 4. With the aid of a diagram, explain the extraction of copper from copper chloride using the method of electrolysis 5. Why can’t aluminium be extracted from its ore in the same way that iron is extracted from iron oxide? ………………………………………………………………………………………………………………………………………………………………. ………………………………………………………………………………………………………………………………………………………………. ………………………………………………………………………………………………………………………………………………………………. ………………………………………………………………………………………………………………………………………………………………. 6. Draw a labelled diagram to represent the bonding in a metal such as Iron deline w 1. 2. 3. 4. 7. Why has the United Kingdom seen a decline in the number of iron and steel works over recent years? ……………………………………………………………………………. ……………………………………………………………………………. ……………………………………………………………………………. ……………………………………………………………………………. …………………………………………………………………………….