Basic Laboratory Techniques
advertisement

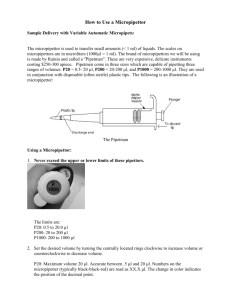
Basic Laboratory Techniques Introduction Scientists use measurements in describing objects and these measurements are based on universally accepted standards. A measurement of height specifies exactly how tall something is, whereas words such as tall or short are open to a wide range of interpretations. In the scientific community, standardized measurements (length, weight and volume) are fundamental to communication. The metric system of measurement is used for all measurement in most countries of the world. The scientific community of the entire world expresses data using the metric system. Therefore, it is necessary for us to know and to be able to effectively use this measurement system. It is also necessary to be able to convert English units into metric units. The metric system units are related to each other by a factor of ten, so interconversions are done by simply moving the decimal point to the left or right. The standard unit of length is the meter (m). The standard unit of mass is the gram (g). The standard unit of volume is the liter (L). For measuring time, the second, minute and hour are units that are used. Names of multiples or fractions of Metric units are formed by adding a prefix to meter, gram or liter. Prefix Symbol Meaning Base Unit Multiplied by teraT trillion 1,000,000,000,000 gigag billion 1,000,000,000 megaM million 1,000,000 kilok thousand 1,000 hectoh hundred 100 decada ten 10 single units, no prefix - Examples: meter, liter, gram 100 decid tenth 0.1 centic hundredth 0.01 millim thousandth 0.001 microu millionth 0.000001 nanon billionth 0.000000001 picop trillionth 0.00000000001 Factor 1012 109 106 103 102 101 10-1 10-2 10-3 10-6 10-9 10-12 VOLUME:WEIGHT CORRELATION: A milliliter is 1/1000 of a liter. This is approximately equal to 1 cubic centimeter (1 cc or 1 cm3). In addition, 1 ml of pure water weighs 1 gram at standard temperature and atmospheric pressure. This is a very convenient conversion (volume:weight) that you should know (i.e. 1 ml water = 1 gram water and 1 l water = 1 mg water). Measuring the volume of a liquid with a graduated cylinder: The surface of a liquid confined in a cylinder curves to form what is known as a meniscus. The meniscus of most liquids curves up the sides of the container, making the center of the curve appear lower than the edges. Since reading the meniscus at the top or at the bottom of the curve will make a difference in the volume measured, it is generally agreed to always read the bottom of the curve. The smaller the container, the greater the curve of the meniscus. The figure to the left is the meniscus in a 10 mL graduated cylinder. Measuring the volume of a liquid with a pipet: Pipets are much more accurate than graduated cylinders. Reading the volume of liquid in a pipet is just like reading a graduated cylinder, however there is one additional technique needed with a pipet. The diameter does not allow a liquid to be poured into a pipet - the liquid must be drawn into the pipet. This picture shows standard pipet pumps used to draw a liquid into a pipet. You may have to practice using the pump with a pipet before you are able to accurately transfer a measured volume of liquid. How to use a pipet pump: • Pour slightly more liquid than needed into a beaker using the "ballpark" graduations on the beaker. Never pipet directly from a reagent bottle. • Gently twist the pipet pump and press it firmly over the top of the pipet. Do not force the pump onto the pipet! You may break the glass pipet. • Place the tip of the pipet below the surface of the liquid in the beaker. • Slowly, draw in more liquid than needed, but do not allow the liquid to enter the pump. Graduated pipets come in a variety of styles: glass, plastic, reusable, disposable, marked for complete delivery and marked for delivery of a specific volume (i.e. you do not fully expel the solution from the pipet). Micropipettors: A micropipettor is essentially a precision pump fitted with a disposable tip. The volume of air space in the barrel is adjusted by screwing the plunger farther in or out of the piston and the volume is displayed on the readout. Depressing the plunger displaces the specified volume of air from the piston; releasing the plunger creates a vacuum, which draws an equal volume of fluid into the tip. The withdrawn fluid is then expelled by depressing the plunger again. Please be sure to take the following precautions: •Never rotate the volume adjustor beyond the upper or lower range of the pipet. •Never use the micropipettor without the tip in place. •Never invert or lay the micropipettor down with a filled tip. •Never immerse the barrel of the micropipettor in fluid. •Never reuse a tip that has been used to measure a different reagent. Measuring mass with an electronic balance: The electronic balance has many advantages over other types of balance. The most obvious is the ease with which a measurement is obtained. All that is needed is to place an object on the balance pan and the measurement can be read on the display to hundredths of a gram. A second advantage, using the Zero button on the front of the balance, is less recognized by beginning science students. Because one must never place a chemical directly on the balance pan, some container must be used. Place the container on the balance and the mass of the container will be displayed. By pressing the Zero button at this point, the balance will reset to zero and ignore the mass of the container. You may now place the substance to be weighed into the container and the balance will show only the mass of the substance. This saves calculation time and effort. However, when the container is removed from the balance, the display will go into negative numbers until the Zero button is pressed again. Accuracy and Precision: The accuracy of an analytical measurement is how close a result comes to the true value. Accuracy refers to the agreement between a measurement and the true or correct value. Determining the accuracy of a measurement usually requires calibration of the analytical method with a known standard. Precision is the reproducibility of multiple measurements and is usually described by the standard deviation, standard error, or confidence interval. Precision refers to the repeatability of measurement. Basic Laboratory Techniques Exercises 1. Complete the following conversions: 0.543 kilograms is equivalent to ________________ grams 1271.72 liters is equivalent to ________________ milliliters 4123.43 centimeters is equivalent to ________________ meters 72.3 milligrams is equivalent to ________________ micrograms 169.3 microliters is equivalent to ________________ milliliters 200 micrometers is equivalent to ________________ centimeters 2. What is the volume of water as seen in the figure for “Measuring the volume of a liquid with a graduated cylinder” ? 3. Practice small volume transfer with adjustable micropipettors. 1) Obtain one of each size micropipettor, compare volume scales, note the maximum volume given on top of each pipettor. Find the volume adjust knob, liquid uptake/dispensing knob and tip eject button. 2) Practice adjusting volume. DO NOT GO OVER THE RANGE OF THE PIPETTOR! Set the 1000 l pipettor at 1000 l, 750 l and 275 l. Note that this pipettor has a numerical readout for the 1000, 100 and 10 places. You may set the pipettor to within 1l using tick marks at the bottom of the scale. Set the 200 l pipettor at 200 l, 150 l and 25 l. Note that this pipettor has a numerical readout for the 100, 10 and 1 places. You may set the pipettor to within 0.1l using tick marks at the bottom of the scale. Set the 20 l pipettor at 20 l, 10 l and 4.5 l. Note that this pipettor has a numerical readout for the 10, 1 and 0.1 places. You may set the pipettor to within 0.01l using tick marks at the bottom of the scale. Large-volume micropipettor: a) Label two microcentrifuge tubes 1 and 2. b) Use the table below as a checklist while adding solutions to tubes 1 and 2. Tube 1 2 Sol. I 100l 150l Sol. II 200l 250l Sol. III 150l 350l Sol. IV 550l 250l c) Set the micropipettor to add appropriate volumes of solutions I-IV to tubes 1 and 2. d) A total of 1000l of colored solutions was added to each tube. To check the accuracy of your measurements, set the micropipettor to 1000l and carefully with draw the solution from each tube. Small-volume micropipettor: a) Label two microcentrifuge tubes 3, 4 and 5. b) Use the table below as a checklist while adding solutions to tubes 3, 4 and 5. Tube 3 4 5 Sol. I 4l 4l 4l Sol. II 5l 5l 4l Sol. III 1l 1l Sol. IV 1l 1l c) Set the micropipettor to add appropriate volumes of solutions I-IV to tubes 3, 4 and 5. d) Sharply tap the tube bottoms on the bench top. Be certain that all of the drops have pooled into on drop at the bottom of the tube. or Place tubes in the microcentrifuge and apply a short pulse of several seconds. Make sure that the reaction tubes are placed in a balanced configuration in the microfuge rotor. Spinning in an unbalanced position will damage the microfuge motor. e) A total of 10l of colored solutions was added to each tube. To check the accuracy of your measurements, set the micropipettor to 10l and carefully with draw the solution from each tube. 3) Practice accurately transferring specific volumes of water with the 1000l and 200l pipettors. 1 ml of DI water weighs 1 g; 1 l of water weighs 1 mg Transfer 1000l of water into a tared beaker on a balance to practice liquid transfer. Repeat four more times. Record the volume (mass) transferred into your notebook. Follow this same procedure with the 200 l pipettor and 200 l of water. Calculate the average volume dispensed for each pipettor. Calculate your % accuracy. % error = (volume wanted – volume dispensed) X 100 volume wanted % accuracy = 100 - % error You should aim for + 1% error. 4. Practice large volume transfer with graduated pipettes. Transfer 10 ml of water with a 10 ml graduated pipette, into a tarred beaker on a balance to practice liquid transfer. Repeat four more times. Record the volume (mass) transferred into your notebook. Calculate the average volume dispensed for the 10 ml pipette. Calculate your % accuracy.