- Society of Photopolymer Science and Technology
advertisement

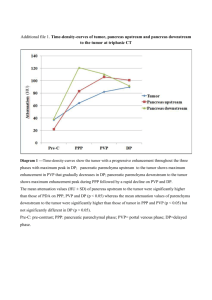
Photocrosslinking System Based on Poly(vinyl phenol) and Fluorene Derivatives with Epoxy Unit Using Photoacid and Photobase Generators Haruyuki Okamura, Chika Harada, Satoshi Morishita, Masamitsu Shirai, Masahiro Tsunooka, Tsuyoshi Fujiki*, Shin-ichi Kawasaki* and Mitsuaki Yamada* Department of Applied Chemistry, Graduate School of Engineering, Osaka Prefecture University, 1-1 Gakuen-cho, Sakai, Osaka 599-8531, Japan * Research & Development Department, Osaka Gas Co., Ltd., 6-19-9 Akanejima, Konohanaku, Osaka 544-0051, Japan Keywords: photocrosslinking, fluorene, epoxide, poly(vinyl phenol), photoacid generator, photobase generator 1. Introduction Photo-induced acid-catalyzed crosslinking systems have been widely studied by many workers [1]. The highly sensitive photocrosslinking materials can be used as negative-type photoresists, coating materials and printing materials. Epoxides and polymers containing cyclic ether units are cationically or anionically crosslinkable. Photocrosslinking of these compounds can be accomplished by the photolysis of initiator molecules, followed by reactions of the resulting photoproducts with cyclic ethers. Photoacid generators and photobase generators can be used as an initiator for the photoinduced crosslinking [2, 3]. In this paper we report the photoinduced insolubilization of polymeric materials based on poly(vinyl phenol) and fluorene derivatives having epoxy moieties as a crosslinker. Photoacid and photobase generators were used as an initiator of insolubilization. The crosslinked materials containing fluorene units are expected to be thermally stable and to show high refractive index. 2. Experimental Poly(vinyl phenol) (PVP) (Mn = 8000) (Aldrich) and triphenylsulfonium triflate (TPST) (Midorikagaku) were used as received. Fluorene derivatives 4,4’-(9-fluorenylidene)bis(phenoxyethyl glycidyl ether) (FBPEG) (softening temperature: ca. 50 oC) and 4,4’-(9-fluorenylidene) bis(phenyl glycidyl ether) (FBPG) (mp: 150 oC) were supplied from Osaka Gas Co., Ltd. O-Phenylacetyl acetonaphtone oxime (PaAnO) was prepared as reported previously. [4] CH2 CH n OH PVP S CF3SO3 TPST O Ch3 CH2 C O N C PaAnO CH2 CH CH2 O CH2 CH O O CH2 FBPG CH CH2 O O CH2 O CH2 O CH2 CH CH2 CH2 CH2 O O FBPEG Scheme 1 O CH2 Sample films (~ 0.2 m) were prepared by casting polymer solutions containing fluorene derivatives and TPST or PaAnO onto quartz plate or silicon wafer. Cyclohexanone and diglyme were used as solvents. Sample films were irradiated at 254 nm using a low-pressure Hg lamp (4 W). Baking of the films was carried out with a conventional hot plate. Irradiated polymer films were developed in tetrahydrofuran and insoluble fraction was determined by comparing the film thickness before and after developments. Thermal decomposition behavior was investigated with Rigaku TAS 100 thermogravimetric analyzer (TGA) under nitrogen flow. 3. Results and discussion When PVP/FBPG (2/1 or 4/1, mol/mol) film containing 2 wt% of TPST was irradiated at 254 nm with a dose of 750 mJ/cm2, no insolubilization was observed. However, the insolubilization was observed when the irradiated films were baked at a given temperature. Fig. 1 shows the relationship between insoluble fraction and post-exposure-bake (PEB) temperature for PVP/FBPG based film irradiated with a dose of 36 mJ/cm2. The sample became insoluble with the increase of PEB temperature. The onset temperature for the insolubilization was dependent on the PVP/FBPG ratio. Insolubilization was not observed for unirradiated samples with heating at 160 oC for 2 min. In Fig. 2 photocrosslinking properties of PVP films and FBPG films with 2 wt% of TPST are shown. Insolubilization did not occur for both films irradiated with a dose of 36 mJ/cm2 and PEB treatment at 160 oC for 2 min. These results indicate that the insolubilization was caused by the reaction of epoxy moieties in FBPG and PVP. Insolubilization of PVP/FBPG films was enhanced by increase of PEB time as well as PEB temperature. Fig. 3 shows the relationship between insoluble fraction and PEB time. Insoluble fraction was observed to be ca. 90% for the sample of the PVP/FBPG (4/1) film with 2 wt % of TPST irradiated with a dose of 36 mJ/cm2 and PEB treatment at 140 oC for 2 min. The dependence of insolubilization of PVP/FBPG films on the irradiated dose is shown in Fig. 4. When the sample film was irradiated with a dose of 10 mJ/cm2 and PEB treatment at 140 oC for 2 min, the insoluble fraction was found to be 90%. Little changes of insoluble fraction were observed at exposure dose above 10 mJ/cm2. On the other hand, insoluble fraction of sample films decreased to 78 % on irradiation with a dose of 140 mJ/cm2 when the PEB treatment was done at 140 o C for 100 80 60 40 20 0 80 100 120 140 PEB temperature ( ℃ ) 160 Fig. 1. Effect of PEB temperature on insolubilization of PVP/FBPG films containing 2 wt% TPST. (○, ●) PVP/FBPG = 4/1, (□, ■) PVP/FBPG = 2/1. Open symbol: Exposed at 254 nm with 36 mJ/cm2. Closed symbol: Unexposed. PEB time: 2 min. Development: THF for 10 min. 100 80 60 40 20 0 80 100 120 140 PEB temperature ( ℃ ) 160 Fig. 2. Effect of PEB temperature on insolubilization of irradiated films containing 2 wt% TPST. (○) PVP/FBPG = 4/1, (□) PVP, (△) FBPG. Exposed dose: 36 mJ/cm2 at 254 nm. PEB time: 2 min. Development: THF for 10 min. 0.5 min. These results indicate that PEB conditions are one of the most important factors for the photoinduced insolubilization of PVP/FBPG/TPST system. The effect of structure of the crosslinkers on the insolubilization efficiency was studied. Fig. 5 shows the relationship between insoluble fraction and PEB temperature for PVP/FBPEG (4/1) and PVP/FBPG (4/1) films containing 2 wt% of TPST o n i r r a d i a t i o n w i t h a d o s e o f 3 6 mJ / c m 2 . 100 80 60 40 20 0 0 1 2 3 4 PEB time (min) 5 Fig. 3. Effect of PEB time on insolubilization of films containing 2 wt% TPST. (○, ●) PVP/FBPG = 4/1, (□) PVP, (△) FBPG. Open symbol: Exposed at 254 nm with 36 mJ/cm2. Closed symbol: Unexposed. PEB temperature: 140 oC. Development: THF for 10 min. 100 80 60 point of FBPEG. FBPEG films containing 2 wt% of TPST irradiated with a dose of 36 mJ/cm2 and PEB treatment above 140 oC became insoluble (Fig. 6), while insolubilization of FBPG films was not observed in the same treatment. It is considered that the reaction for insolubilization of PVP/FBPEG films was the crosslinking of epoxy moieties of FBPEG with PVP and the photoinduced acid-catalyzed polymerization of epoxy moieties in FBPEG occurred simultaneously. The thermal degradation of the crosslinked PVP/FBPG films was observed at about 300 oC by TGA analysis, which suggests that crosslinked PVP/FBPG films have good thermal stability. Crosslinked PVP/FBPG films showed slightly higher refractive indices (nD : 1.61 ~ 1.62) compared to that of PVP (nD : 1.60). It is known that epoxy derivatives act as a crosslinker of phenol-novolac resin in the presence of tertiary amines or KOH [5]. However, no attempt has been made for photoinduced base-catalyzed crosslinking system of epoxy derivatives and PVP. PaAnO is known to generate benzyl amine on irradiation at 254 nm (Scheme 2). The insolubilization was observed for PVP/FBPEG (4/1) film containing 10 wt% PaAnO, when irradiated at 254 nm with a dose of 200 mJ/cm2 and PEB treatment above 140 oC for 5 min. Fig. 7 shows the relationship between insoluble 40 20 100 0 0 20 40 60 80 100 120 140 Exposure dose (mJ/cm2) Fig. 4. Effect of irradiation dose on insolubilization of irradiated films containing 2 wt% TPST. (○, ●) PVP/FBPG = 4/1, (□) PVP, (△) FBPG. Open symbol: PEB treatment at 140 oC for 2 min. Closed symbol: PEB treatment at 140 oC for 0.5 min. Development: THF for 10 min. Insolubilization of PVP/FBPEG film occurred with the PEB treatment at lower temperature compared to PVP/FBPG film. Insolubilization of unirradiated PVP/FBPEG films with heating at 160 o C for 2 min did not occur. The lower PEB treatment temperature for the PVP/FBPEG system is due to the high reactivity of the epoxy moiety in FBPEG due to its flexibility and lower melting 80 60 40 20 0 70 100 130 PEB temperature ( ℃ ) 160 Fig. 5. Effect of fluorene derivative structure on insolubilization of films containing 2 wt% TPST. (○, ●) PVP/FBPG = 4/1, (□, ■) PVP/FBPEG = 4/1. Open symbol: Exposed at 254 nm with 36 mJ/cm2. Closed symbol: Unexposed. PEB time: 2 min. Development: THF for 10 min. 100 CH2 80 C O O N 60 h CH2 CO2 N C CH2 CH2 - CO2 Ch3 H2O NH2 + O Ch3 C Ch3 N C C Ch3 40 20 Scheme 2 0 70 100 130 PEB temperature ( ℃ ) 160 Fig. 6. Insolubilization PVP/FBPEG and FBPEG films containing 2 wt% TPST. (○, ●) FBPEG, (□, ■) PVP/FBPEG = 4/1. Open symbol: Exposed at 254 nm with 36 mJ/cm2. Closed symbol: Unexposed. PEB time: 2 min. Development: THF for 10 min. 100 80 60 40 20 0 130 140 150 160 170 PEB temperature (℃ ) 180 Fig. 7. Insolubilization of PVP/FBPEG (4/1) films containing PaAnO. PaAnO: (○, ●) 5 wt%, (□, ■) 10 wt%. Open symbol: Exposed at 254 nm with 200 mJ/cm2. Closed symbol: Unexposed. PEB time: 5 min. Development: THF for 10 min. fraction and PEB temperature for PVP/FBPG based film irradiated with a dose of 200 mJ/cm2. The insoluble fraction increased with PEB temperature. The onset temperature for the insolubilization was dependent on the amount of PaAnO added. PVP/FBPEG (4/1) film containing 10 wt% PaAnO became insoluble on heating above 170 oC. Decomposition of PaAnO was observed at 192 oC by TGA measurement. This insolubilization may be due to the decomposed compound of PaAnO at 170 oC. When the amount of PaAnO was reduced to be 5 wt% in films, the insolubilization was observed for the irradiated film with PEB treatment above 140 o C. Insolubilization did not occur for the samples without irradiation on PEB treatment at 180 oC for 5 min. These results indicate that photoinduced base-catalyzed crosslinking system of PVP/BPEFG requires the proper content of PaAnO. PEB condition is also important to enhance the insoluble fraction of PVP/FBPEG/PaAnO system. In the case of PVP/FBPEG film containing 5 wt% of PaAnO, insoluble fraction reached to 90% after irradiation (200 mJ/cm2) and PEB treatment at 150 o C for 8 min. References 1. J. P. Fouassier, J. F. Rabeck, Radiation Curing in Polymer Science and Technology, Elsevier Applied Science, New York (1993). 2. M. Shirai, M. Tsunooka, Prog. Polym. Sci., 21 (1995) 1. 3. M. Shirai, M. Tsunooka, Bull. Chem. Soc. Jpn., 71 (1998) 2483. 4. K. Ito, Y. Shigeru, Y. Kawata, K. Ito, M. Tsunooka, Can. J. Chem., 73 (1995) 1924. 5. D. Gagnebien, P. J. Madec, E. Marechal, Eur. Polym. J., 21 (1985) 273.