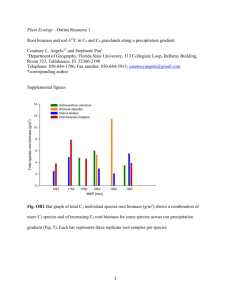
- IGCP 588 Preparing for Coastal Change
advertisement

Variability of bulk organic δ13C and C/N in the Pearl River delta and estuary, southern China and its indication for sources of the estuarine sediment Fengling Yua*, Yongqiang Zongb, Jeremy M. Lloyda, Guangqing Huangc, Melanie J. Lengd,e, Christopher Kendrickd, Angela L. Lambd, Wyss W.-S Yimb a Department of Geography, University of Durham, South Road, Durham DH1 3LE, UK Department of Earth Sciences, The University of Hong Kong, Pokfulam Road, Hong Kong SAR, China c Guangzhou Institute of Geography, 100 Xian Lie Road, Guangzhou 510070, China d NERC Isotope Geosciences Laboratory, British Geological Survey, Keyworth, Nottingham, NG12 5GG, UK e School of Geography, University of Nottingham, Nottingham NG7 2RD, UK ____________________________________________ *Corresponding author. Tel: +44 (0)191 3341817; Fax: +44 (0)191 3341801; Email address: fengling.yu@durham.ac.uk b Abstract This paper presents carbon isotope composition (δ13C) and C/N of organic matter from source to sink in the Pearl River catchment, delta and estuary, and discusses the applicability of δ13C and C/N as indicators for sources of estuarine sediment. In addition to the 91 estuarine surface sediment samples, other materials collected in this study cover the main sources of organic material to estuarine sediment. These are: terrestrial organic matter (TOM), including plants and soil samples from the catchment as well as mangrove leaves and sediment from the salt marshes; estuarine and marine particulate organic carbon (POC) from both summer and winter. Results show that average δ13C of estuarine surface sediment increases from –25.0 ±1.3‰ from the freshwater environment to –21.0 ±0.2‰ from the marine environment, with C/N decreasing from 15.2 ± 3.3 to 6.8 ±0.2. In the source areas, C3 plants have lower δ13C than C4 plants of –29.0 ±1.8‰ and –13.1 ±0.5‰ respectively. δ13C increases from –28.3 ±0.8‰ in the forest soil to around – 24.1‰ in both riverbank soil and mangrove soil due to increasing proportion of C4 grasses. The δ13CPOC increases from –27.6 ±0.8‰ in the freshwater areas to –22.4 ±0.5‰ in the marine-brackish-water areas in winter, and ranges between –24.0‰ and –25.4‰ from freshwater areas to brackish-water areas in summer. Comparison of the δ13C and C/N between sink and source indicates a weakening TOM and freshwater POC input in the surface sedimentary organic matter seawards, and a strengthening contribution from the marine organic matter. Thus we suggest that the bulk organic δ13C and C/N can be used as indicators for sources of the sedimentary organic matter. Organic carbon in surface sediments derived from anthropogenic sources such as human waste, organic pollutants from industrial and agricultural activities accounts for less than 10% of the total organic carbon (TOC). Although results also indicate elevated δ13C of sedimentary organic matter due to some agricultural products such as sugarcane, C3 plants are still the dominant vegetation type in this area, and the bulk organic δ13C and C/N is still an effective indicator for sources of estuarine sediments. Keywords: δ13C, C/N, sediment source, Pearl River, estuary, southern China -2- 1. Introduction Preservation of organic matter in estuarine and coastal sediments is an important process in the global carbon cycle as more than 90% of the carbon buried in the oceans occurs in continental margin sediments (Emerson and Hedges, 1988; de Haas et al., 2002). In estuarine areas, organic matter can be supplied both from autochthonous sources (such as plants growing on the sediment surface) and allochthonous sources (organic material transported predominantly by the tide or a river). Better constraints on the sources of organic matter in marine sediments are needed to understand the processes responsible for its preservation (Mayer, 1994). Such understanding will help estimate the possible contribution of different sources of organic matter to marine organic matter, relating to the global cycle of carbon (Schlunz et al., 1999; Gaye et al., 2007). Bulk organic δ13C and C/N have been widely used to elucidate the source and fate of organic matter in the terrestrial, estuarine and coastal regions (e.g. Hedges and Parker, 1976; Fontugne and Jouanneau, 1987; Goñi et al., 1997; Bianchi et al., 2007; Middelburg and Herman, 2007; Harmelin-Vivien M. et al., 2008; Ramaswamy et al., 2008) and also as proxies in palaeoclimatic studies (e.g. Chivas et al., 2001; Lamb et al., 2006; Zong et al., 2006; Wilson et al., 2005; Mackie et al., 2007). The Southeast Asian area is one of the most densely-populated areas in the world, as well as one of the most industrialized regions. The contribution of organic carbon from land to the ocean via major fluvial systems in this area has not been well estimated. Some studies have been carried out to assess the contribution of terrigenous organic matter to the South China Sea (e.g. Hu et al., 2006), as well as a number of studies investigating the modern-day organic carbon isotopic signature from the Pearl River delta and estuary (Dai et al., 2000; Chen et al., 2003, 2004; Jia and Peng, 2003; Callahan et al., 2004; Zong et al., 2006). However, a detailed understanding of the range of sources of organic matter and their relative contribution to the bulk sediment organic carbon within an estuary is still rather poorly constrained. Detailed investigation of δ13C and C/N of the organic matter through the full estuarine complex (from freshwater to marine environments) is one way to address this problem. Here we aim to use this technique to establish the full range of δ13C and C/N of the organic matter in the Pearl River estuary from source to sink. We will then assess the suitability of this proxy as an indicator for dominant vegetation type, sediment source and environmental conditions as well as the role of anthropogenic influence on the δ13C and C/N in the Pearl River estuary. 2. Materials and methods 2.1. The Pearl River delta and estuary The Pearl River delta is located at 21°20´-23°30´N and 112°40´-114°50´E (Fig. 1a), and formed during the last 9000 years (Zong et al., 2009). The Pearl River (Zhujiang) is the general name for the three rivers (the East, the North and the West, Fig. 1a) that flow into the Pearl River delta-estuary before entering the South China Sea. It is the second largest river in China in terms of water discharge (about 330 ×109 m3/year; Hu et al., 2006). The River is 2214 km in length (the West River), and it drains an area of 425,700 -3- km2 (Li et al., 1990). The suspended sediment concentration in the Pearl River is relatively low compared with other major Asian rivers (Zong et al., 2009), with a mean concentration of about 0.172 kg m-3 and an annual flux of about 30.64 106 t (Wai et al., 2004). About 92-96% of the suspended sediment is discharged during the wet season (from April to September, monsoon summer), with maximum river discharge occurring in July. The warm/hot wet season is followed by a cool/cold dry season (monsoon winter from October to March). Approximately 80% of sediment influx to the estuary is deposited within the Pearl River estuary, the remaining being transported to the South China Sea (Xu et al., 1985). The coastal waters in the vicinity of the Pearl River estuary are influenced by three water regimes: the Pearl River discharge, oceanic waters from the South China Sea and coastal waters from the South China Coastal Current (Morton and Wu, 1975; Yin et al., 2004). These water regimes are subjected to two seasonal monsoons. In winter, the northeast monsoon prevails, the China Coastal Current dominates the coastal waters of Hong Kong, and freshwater discharge is at its lowest. In summer when the monsoon blows from the southwest direction and the Pearl River discharge reaches the maximum, the interaction of the estuarine plume and oceanic waters from the South China Sea dominate the coastal water regime (Yin et al., 2004). The Pearl River delta is within the tropical climate zone, with mean annual temperature ranging from 14 to 22°C across the basin and precipitation ranging from 1200 to 2200 mm year-1 (Zhang et al., 2008). Subtropical and tropical forests are the dominant vegetation type in the Pearl River catchment (Winkler and Wang et al, 1993). The natural plants in this area are dominantly C3 plants, mixed with some C4 grasses, while sugarcane (a C4 plant) is one of the major agricultural products in the lower deltaic area today. 2.2. Filed data collection 2.2.1. Terrestrial organic matter Terrestrial organic matter (TOM) includes land plants and soil organic matter (Fig. 1b; Table 1, 2). Plant samples collected include: 1) C4 plants, including general C4 grasses, such as Panicum maximum Jacq. and sugarcane (Saccharum officinarum). As this study also examines δ13C and C/N of agricultural plants, it is necessary to separate sugarcane from other non-agricultural grasses. The ‘general C4 grasses’ are the non-agricultural, naturally-grown C4 grasses in this area; and 2) C3 plants, including general C3 plants (e.g. Pteris semipinnata, Pinus sp.), mangrove (Avicennia, Kandelia obovata, Bruguiera gymhorrhiza, Acanthus ilicifolius, Aegiceras corniculatum, Avicennia marina, Ficus microcarpa) and agricultural plants e.g. banana (Musa acuminata), lotus (Nelumbo nucifera), reed (Phragmites australis) and rice (Oryza sativa). Here, the ‘general C3 plants’ are the non-agricultural, naturally-grown C3 plants in this area. Representative plant organs were collected and initially stored in paper bags until dried at 50ºC over night in an oven. Soil samples were collected from c. 3 cm depth from under the surface (to avoid fresh plant roots and disturbed soil, Liu et al., 2003), and stored in polyethylene tubes in a fridge before sample preparation and analysis. As both C3 and C4 plants grow in the terrestrial area, organic matter in terrestrial soil samples is a mixture of both kinds -4- of plants. Soil samples are clustered into the following groups: forest soil, mangrove soil, riverbank soil and agricultural soil, depending on sample location as well as the dominant vegetation type. Plants and soil samples were collected in June 2006, except for agricultural plants (banana, lotus, reed, rice and sugarcane) and agricultural soil samples which were collected in August 2007. 2.2.2. Estuarine organic material Estuarine organic material includes seasonal particulate organic carbon (POC; mainly derived from freshwater or marine phytoplankton and terrigenous organic matter) and estuarine surface sediments (Fig. 1b; Table 3, 4; Table 5). A total of 49 POC samples (PE41-92, without PE76-77) were collected in winter (December 2006), and 45 POC samples (PE41-75) in summer (June 2006) including 35 samples on filter paper and 10 samples using a 70 µm net. POC samples were collected by filtering water samples using the fibreglass filter paper (Fisher Brand MF200) in the laboratory. POC samples were also collected using a 70 µm net from some sites in June 2006 (no POC on net was collected during winter due to small size of the boat). Samples were then washed from the net into a sampling tube, and refrigerated prior to analysis. A total of 91 surface sediment samples (PE1-PE92, without PE43; Fig. 1b) were obtained using a grab sampler from a boat. The top 10 cm sediment was collected, which potentially represent sediment deposited during the past 6-10 years, according to the sedimentation rate of around 0.8 cm/year on shoals within the estuary (Li et al., 1990). Samples were sealed in polyethylene tubes and stored in fridge at 2-3ºC before analysis. A total of three field campaigns were carried out to collect these surface sediment and POC samples. Surface sediment samples PE01-40 were collected by Zong et al. (2006) in June 2005. Surface sediment sample PE41-77 were collected by this study in June 2006, and PE78-92 were collected in December 2006. Summer POC samples PE41-75 were collected in June 2006 and winter POC samples PE78-92 were collected in December 2006. 2.2.3. Estuarine environmental variables Environmental variables including water salinity (Sal), water depth (WD), pH, total dissolved solid (TDS) and water temperature (Temp), were measured from the same sampling sites as the POC samples (Fig. 1b; Table 3). Summer water salinity and mean water depth from PE78-PE92 were interpolated based on a bathymetry map of the area. Mean water salinity for sites of PE41-PE92 is the mean value of seasonal values measured. Water salinity was measured using the Practical Salinity Scale. Mean water salinity and mean water depth from PE1-PE40, PE76 and PE77 were interpolated based on bathymetry maps of this area. These variables were measured at 50 cm below water surface using a YSI meter. 2.3. Laboratory methods 2.3.1. Sample preparation -5- Soil and sediment samples were prepared using 100 ml 5% HCl to remove carbonates. They were then washed 3 times with deionised water using the fibreglass filter paper, before being dried down at 50ºC overnight, homogenised in a pestle and mortar, and weighed (25-50 mg) for δ13C and C/N analysis. Plant samples are placed in a –80ºC freezer overnight followed by freeze drying for 24 hours. These samples were then crushed and homogenised in a ball mill at a speed of 500 rpm for 5-15 minutes (depending on type of plants) and then weighed (1-2 mg) for δ13C and C/N. POC samples on the filter paper were dried in the field, and were difficult to remove from filter papers. Some were successfully removed by gently scrapping off the filters while others that could not be removed had to be analysed while attached to the filters. The POC on the filters could only be measured for C/N ratios (rather than TOC and TN) as absolute weights could not be measured (Table 3). 2.3.2. Sample analysis Carbon isotopes and C/N analyses were performed by combustion in a Carlo Erba NA1500 (Series 1) on-line to a VG Triple Trap and Optima dual-inlet mass spectrometer, with δ13C calculated to the VPDB scale using a within-run laboratory standards (BROC) calibrated against NBS-19 and NBS-22. Replicate analysis of well-mixed samples indicated a precision of ±<0.1‰ (1SD). C/N was determined by reference to an Acetanilide standard. Replicate analysis of well-mixed samples indicated a precision of ±<0.1%. The isotopic ratios are expressed as δ13C, in units of per mille (‰). Organic matter is depleted in 13C relative to V-PDB, so δ13C of organic material is negative. The weight ratio of total organic carbon (%TOC) to total nitrogen (%TN), giving C/N is usually measured along side δ13C and help to distinguish carbon sources. Particle size analysis was carried out using a laser granular meter (Coulter LS 13200). Values of the δ13C, C/N, particle size and environmental parameters in this paper are presented in the format of ‘average value ±standard deviation (SD)’. Values of δ13C or C/N of some samples are not available due to low content of organic matter in those samples. 3. Results 3.1. Hierarchical Cluster Analysis All 92 samples were subjected to Hierarchical Cluster Analysis (using SPSS for Windows 15.0) based on winter salinity and sand concentration. The cluster analysis defined 9 groups (G1-G9) with a clear geographical control (illustrated in Fig. 2; Table 5). Surface sediment samples are from the 91 sampling sites (without site PE43) covering G1-G9. The POC samples are only from the western estuary where suspended sediment concentration was high enough to allow measurements to be made, with samples ranging from G2-G6 for winter POC and G2-G5 for summer POC. The 9 groups defined by selected environment variables clearly highlight a trend from the full-freshwater environment (G1-G3) to the full-marine environment (G8-G9), via brackish areas (G4-G7) (Fig. 2; Table 6). The freshwater groups have low annual -6- average water salinity of 3.4±3.6, which increases to 15.8±5.3 and 28.4±3.1 in the brackish-water areas and reaches 32.9±0.8 under the full marine environment (Table 6). Mean water depth is lower than 5 m in the freshwater area, getting deeper seawards, and becomes deeper than 20 m in the shallow marine area. Meanwhile, average particle size gets finer offshore with a reduction in sand component. All variables tend to be more stable in the marine area than in the freshwater area (Table 6). In addition to the spatial diversity of the environmental conditions, seasonal variability is also observed. For G2-G5, mean winter salinity is 14.0±11.6, ranging between 0.1 and 33.9. This is significantly higher than that in summer of 3.1±4.8, ranging between 0.1 and 18.2 (Table 3). A similar situation is found in the concentration of total dissolved solids (TDS) of 15.5±12.1 g l-1 in winter compared to 2.0±2.7 g l-1 in summer. Areas under the freshwater- and fresh-brackish conditions are more sensitive to seasonal changes than those under marine- and marine-brackish conditions, as seasonal variation of these environmental parameters are larger for G2 than G5. 3.2. Terrestrial organic matter 3.2.1. Plants Plant samples collected in this study are composed of two main groups: C3 plants (dominant vegetation in the Pearl River delta), and some C4 plants. Results show that C3 plants in general have lower δ13C with an average of –29.0±1.8‰ compared to C4 plants with an average of –13.1±0.5‰ (Fig. 3a). C3 plants generally have the lowest δ13C of – 29.9±1.3‰, followed by the agricultural C3 plants (e.g. rice, lotus and banana) and mangroves, with δ13C values of –28.2±1.4‰ and –27.1±1.7‰ respectively (Fig. 3b). C4 plants (mostly grasses) have δ13C of –13.2±0.5‰, slightly lower than sugarcane which has δ13C of –12.7±0.2‰ (Fig. 3b). C/N of C3 and C4 plants are widely variable (7.4-61.8 for C3 plants and 8.2-40.3 for C4 plants) and overlap significantly, with the average value of 22.7±11.6, and 24.6±9.4 respectively (Fig. 3a). It is impossible to distinguish between the two plant types based on C/N alone. Within the C3 plants, agricultural plants have lower C/N (13.7±2.7) than other C3 plants (21.7±10.7) and mangroves (31.0±12.5). Within the C4 plants, sugarcane has a slightly higher C/N of 30.6±8.4 compared to other C4 plants of 21.5±8.9 (Fig. 3b). 3.2.2. Terrestrial soil Terrestrial soil samples have δ13C between –28.9‰ and –19.3‰, and C/N between 7.7 and 20.4, with a negative correlation between δ13C and C/N (Fig. 4a). As expected soil samples from different areas of vegetation cover, have different δ13C and C/N values. Soil samples from pine forest have the lowest δ13C of –28.3±0.8‰, but the highest C/N of 17.9±3.6. Soil samples from riverbanks and mangrove areas have similar δ13C and C/N, around –24.1‰ and 12.5 respectively, although both δ13C and C/N are relatively more variable in the mangrove soil than in the riverbank soil (Fig. 4b). Soil samples from farmlands have the highest δ13C of –21.7±0.7‰ and the lowest C/N of 8.9±1.1 (Fig. 4b). 3.3. Estuarine particulate organic carbon -7- 3.3.1. POC collected on filter paper Overall, δ13C of POC shows very little if any seasonal variations, the average δ13C of POC in summer (–24.5‰) is within error of winter (–24.2‰) (there is slightly more variation in winter (SD=1.9) than in summer (SD=1.0)). C/N of the winter POC ranges between 6.0 and 9.0, with a mean value of 7.1±0.9. It is slightly lower than the summer POC, ranging between 8.0 and 10.2, averaging 9.2±1.1 (Fig. 5). δ13C and C/N of POC varies between groups from different environments. In winter, freshwater POC has the lower δ13C (–27.6±0.8‰, G2) than the marine-brackish POC (– 22.4±0.5‰, G6), while samples from the fresh-brackish water area have δ13C between the two (–24.5±1.3‰ for G4; –23.6±1.2‰, G5) (Fig. 6a). Variation in C/N of winter POC between groups is not as significant as δ13C (Fig. 6a). In summer, the average δ13CPOC of each group is similar and does not show any trend between groups. Differences in both δ13C and C/N of summer POC is not significant (Fig. 6b). δ13C and C/N of POC show good correlation between salinity and other environmental conditions (Fig. 6c, d), especially in the winter. In winter, δ13C increases from –27.6‰ in the freshwater area further inland to around –21.6‰ in the marine-brackish area near the estuary mouth, with increasing salinity (Fig. 6c). Correlation between C/N and environmental variables is not as significant as that of δ13C. C/N is found to be high in the middle estuary (7.6±1.0, G5) and decreases landwards and seawards (<7.0). In summer, both δ13C and C/N are more uniform than winter and not clearly correlated to the environmental variables (Fig. 6d). 3.3.2. POC collected by a 70 µm net Estuarine POC is composed of organic carbon from fine-size plankton and relatively coarse TOM. As the size of plankton (e.g. diatoms) is usually smaller than 80 μm, organic matter collected on filter paper has a higher proportion of plankton/algae, while those collected in a 70 µm net presents higher proportion of TOM over plankton. Results show that the POC samples from the 70 µm net have a lower average δ13C of – 25.6±1.0‰, compared to the filter paper sample from the same location (i.e. –24.3±1.0‰ in summer and –24.2±1.5‰ in winter, Fig. 7). The average C/N of the POC from the net samples is 10.7±4.5, which is higher and more variable than that of the POC collected on filter paper of 9.4±1.1 in summer and 7.8±1.0 in winter respectively (Fig. 7). POC values from the samples collected using the net fall into two groups. POC from locations further seaward, such as PE65, PE67, PE68, and PE71 (Fig. 1b), tend to have lower δ13C and C/N than POC on filter paper from both seasons, while those from the inner estuarine area have similar δ13C with the POC from the filter papers but higher C/N (Fig. 7). 3.4. Estuarine surface sediment The estuarine surface sediment samples have δ13C ranging from –27.0‰ to –20.8‰ and C/N ranging between 20.1 and 6.5, with δ13C and C/N negatively correlated (Fig. 8a). With the environmental change from the full-freshwater environment to the full-marine environment from G1 to G9, a clear trend is observed in both δ13C and C/N values. δ13C -8- becomes progressively higher from G1 to G9, increasing from –25.0±1.3‰ in G1 to – 21.0±0.2‰ in G9 (Fig. 8), with increasing water salinity and decreasing sand concentration (Table 5). Meanwhile, C/N also shows a clear trend decreasing from 15.2±3.3 in G1 to 6.8±0.2 in G9. G3 does not fit in this trend with δ13C of –23.0±1.6‰ and C/N of 11.1±1.3, which is lower than G4 (Fig. 8b). Both δ13C and C/N are more stable in the marine environment compared to the freshwater (Table 5). 4. Discussion 4.1. Terrestrial organic matter and its sources Plants from the study area can be placed in two main groups, C3 and C4 plants, based on the way they fix carbon from atmospheric CO2 during the photosynthetic process. This process produces significant difference in the δ13C signature of the organic carbon from these two groups of plants. C3 plants use the Calvic-Benson cycle (C3 pathway) (Craig, 1953; Park and Epstein, 1960), through which they absorb and conserve 12C from atmospheric CO2 more effectively compared to the Hatch-Slack pathway used by C4 plants (Hatch and Slack, 1970). This results in greater discrimination against 13C in C3 plants than C4 plants, with C3 plants producing lower δ13C around –28.1‰ (Graig, 1953; Fry and Sherr, 1984; O’Leary, 1985) than C4 plants with values around –13.0‰ (Bender, 1971; Fry and Sherr, 1984; Emerson and Hedges, 1988). The C3 plants analyzed in this study have an average δ13C of –29.9‰ (Fig. 3), about 1.8‰ lower than values reported by other studies. This might be due to the high precipitation in the Pearl River delta area, with precipitation over 1500 mm/yr (Li et al., 1990). A similar case has been reported by Austin and Vitousek (1998) where δ13C of C3 plants shifts from –29.9‰ to –25.6‰, when the sampling site changed from an area of 5000 mm/yr precipitation to an area of only 500 mm/yr. The average δ13C of C4 plants from this study is –13.1‰ (Fig. 3), similar to other studies (e.g. Bender, 1971; Fry and Sherr, 1984; Emerson and Hedges, 1988). Terrestrial soil is one of the direct sinks of the organic carbon from the surface vegetation, and its organic carbon signature depends greatly on the dominant vegetation type. This explains the variation in organic carbon isotope signatures found in soil samples from different areas of the Pearl River delta, e.g. forest, riverbank and tidal flats (Fig. 4). Soils from the forest mainly receive organic carbon from C3 plants (e.g. pine), and tend to have low δ13C of –28.3±0.8‰, but high C/N of 17.9±3.6 (Fig. 4b). Riverbank soil samples are from the flood plain along the North River, dominated by shrubs and grass. The increasing proportion of C4 grass on the flood plain produces relatively higher δ13C in the riverbank soil (–24.1±1.0‰) compared to the forest soil (Fig. 4b). Similar δ13C and C/N are found in the riverbank and mangrove soil (–24.0±1.9‰) and are due to the similar proportion of C4 plants growing on the flood plain and the tidal flat. Other studies also report the δ13C of soils from C4-dominated areas as being around –21.0‰, and around −24.0‰ from areas mixed by C3 and C4 plants (Driese et al; 2005; Saia et al. 2008). Many studies have used δ13C of terrestrial soil to reflect different vegetation types (Mackie, et al., 2005; Fan et al., 2007; Cao et al 2008; Driese et al., 2008; Saia et al., 2008). Usually, C/N of soil organic matter is lower than 20. Although the range of C/N is -9- not as wide as the C/N of either C3 or C4 plants, it is still impossible to use C/N alone to distinguish different sources of the TOM. 4.2. Estuarine particulate organic carbon and its sources In the Pearl River delta, terrestrial organic matter enters the river system in the form of plant fragments and soil organic matter, and mixes with in situ (freshwater, brackishwater, marine) plankton and algae within the estuary. Accordingly, within the estuarine area, the POC receives carbon from the TOM and in situ aquatic productivity (freshwater, brackish-water or marine plankton). The terrigenous freshwater POC, having δ13C around −28.7‰ (Fig. 5 and 6), is delivered into the estuarine system by the river runoff. It dilutes the signal produced by the brackish-water and marine POC which usually has δ13C around −21.2‰ and C/N ≤ 11.0 (Fig. 5 and 6). Higher δ13C in marine POC compared with riverine POC has also been reported from other coastal areas (Middelburg and Nieuwenhuize, 1998; Countway et al., 2006; Bianchi et al., 2007; Middleburg and Herman, 2007; Zhang et al., 2007; Bird et al., 2008). Wu et al. (2007) report δ13C of the POC varying from –24.4‰ in the Yangtze River to –21.0‰ on the East China Sea Shelf. Middleburg and Herman (2007) suggested that the correlation between δ13CPOC and salinity is more significant in river-dominated estuaries, such as the Rhine estuary and the Douro estuary, than in tidal-dominate estuaries such as the Gironde estuary and the Loire estuary, Western Europe. In the Pearl River estuary, the balance between TOM and in situ plankton in the estuarine POC is strongly influenced by the strength of the freshwater runoff. It is, therefore, the strength of runoff that appears to control the spatial and temporal variation of the carbon isotopic signature of the estuarine POC. In winter, significant reduction in monsoonal precipitation results in a remarkable reduction in the TOM input into the estuary, and therefore the POC within the estuary is dominated by in situ phytoplankton (e.g. diatoms, dinoflagellates, green algae, euglenoides). δ13C of the winter POC shows a clear trend from freshwater areas (around –28.0‰) to the marine-brackish areas (around –22.0‰), reflecting the variation in δ13C and C/N of plankton under different environments (Fig. 6a). In the Pearl River estuary, δ13C of the freshwater POC typically ranges from –30‰ to –25‰, and the brackish POC typically ranges from –22‰ to –26‰ (Fig. 6c, d) which is also reported by other studies (Lamb et al., 2006; Middelburg and Herman, 2007; Tesi et al., 2007). The low C/N (7.1±0.9) of winter POC supports the assumption that winter POC is dominated by plankton, as the phytoplankton tends to have low C/N of 5-7 (Lamb et al., 2006). In summer the high freshwater flux brings larger amounts of TOM into the estuary (clearly seen in the amount of sediment collected by the filter in the summer compared to the winter in this study) resulting in a higher proportion of TOM in summer POC, suggested by the relatively higher C/N. At the same time the influence of the strong freshwater flux extends further seawards of the estuary, and produces a relatively uniform mix of POC within the estuary. This mixing and homogenisation of the POC throughout the estuary reduces the spatial variability in δ13C of the summer POC. This is indicated by the considerable overlap in δ13C and C/N of the summer POC from groups G2 to G5 across the estuary (Fig. 6b). The POC collected with the 70 µm net is assumed to have a higher proportion of plant fragments than the POC collected on the filter paper as a higher proportion of the - 10 - relatively smaller phytoplankton can pass through the net, but are trapped on the filter. The POC from the net would, therefore, be expected to record (on average) lower δ13C and have higher C/N than the POC from the filter paper. This is indeed the case (Fig. 7) with POC samples from the net having an average δ13C of –25.6‰ and C/N of 10.7 compared to values of –24.3‰ and 9.5 for samples from the filter paper. Previous studies suggest that the range of δ13CPOC is small in estuaries of high turbidity, between –24‰ and –26‰ (Fontugne and Jouanneau, 1987; Tan et al., 2004), which reflects the dominance of terrigenous POC input into the estuary over other sources. This further supports the suggestion that the proportion of TOM in the POC is one of the main factors influencing δ13C and C/N of POC, while the strength of the freshwater flux controls seasonal and spatial distribution of the TOM in the POC. However, during periods of reduced freshwater flux (during winter in the Pearl River estuary area for example) in situ productivity will tend to be the dominant source of POC and, hence, be the important control on spatial variability of δ13C of POC (illustrated by winter distribution of δ13C, Fig. 6a). 4.3. Estuarine surface sediment and its sources Comparison of δ13C and C/N from a wide range of potential sources of sediment to the Pearl River estuary with measurements of bulk sediment samples from the estuary makes it possible to identify the dominant sources of organic sediment to the estuary floor. Organic matter within the surface sediment is mainly composed of the POC, especially from the marine surface sediment (Liu et al., 2007), while the organic matter of the surface sediments from freshwater environments is a combination of freshwater in situ plankton productivity and the TOM. Spatially, δ13C increases from –25.0‰ to –21.0‰ from river tributaries to marine areas with C/N decreasing from 15.2 to 6.8. This trend indicates a decreasing TOM contribution into the surface sediment organic matter, with increasing POC contribution seawards. Inner estuarine areas near the river mouth predominantly receive TOM delivered by the freshwater runoff. The proportion of TOM in the surface sedimentary organic matter decreases seawards as the influence of the freshwater runoff becomes weaker. In the marine area, surface sedimentary organic matter is dominated by the POC as TOM flux is weakened by the tide influence. Applicability of δ13C and C/N of the surface sediment for indicating sources of the sediment has been explored in previous studies in the estuarine and continental areas based on the sedimentation rates (Hedges and Parker, 1976; Goñi et al.,. 1997; Hu et al., 2006; Ramaswamy et al., 2008). Hu et al. (2006) examined organic carbon, total nitrogen, δ13C and δ15N of surface sediments from the Pearl River estuary and adjacent shelf, suggesting proportionally higher terrestrial-derived organic carbon at the river mouth and the western coast compared to marine-origin organic carbon. Using a mixing model (Schultz and Calder, 1976), Hu et al. (2006) further suggest the algal-derived organic carbon content is estimated to be low (≤0.06%) at the river mouth and higher (up to 0.57%) on the adjacent inner shelf. A decreasing contribution of TOM in the surface sediment organic matter is also suggested by Zong et al. (2006) by examining the bulk organic δ13C and C/N of the surface sediment, as well as diatom records. Detailed bulk organic δ13C and C/N of the organic matter from potential source areas of the surface sediment in the Pearl River estuary investigated in this study support the - 11 - spatial distribution of the TOM and POC in the estuarine area. Different dominant organic matter sources between the freshwater and marine environments generate the lower δ13C but higher C/N in the freshwater sediment compared to the marine sediment (Fig. 8b). Surface sediments from the freshwater area of this study have low δ13C (e.g. – 23.9‰ for surface sediment and –26.3‰ for the POC on average in G2). Surface sediments from the brackish/marine environment, with little freshwater influence, have high δ13C (e.g. −21.0‰ in G9, Fig. 8b) due in part to higher δ13C of the marine POC (– 24.0‰ on average in G5 and –22.4‰ for G6, Fig. 6). C/N is useful for separating algae and TOM, with algae having C/N between 5 and 8, while TOM has C/N typically higher than 15 (Meyers, 1997). Low C/N of the surface sediments at the marine end (e.g. 6.9 for G9, Fig. 8b) suggests higher organic matter contribution from marine algae (Hu et al., 2006; Wu et al., 2007). Factors that influence the organic carbon isotopic signature of POC, therefore, also become significant for the signature of the surface sediment, such as strength of the freshwater flux and hydrological conditions. The contribution of marine algae to the sedimentary organic carbon is limited by the turbidity and nutrient content of the water column (Yin et al., 2004; Huang et al., 2003; 2004). Turbidity is higher at the river mouth (nearer the source of sediment flux), the contribution of in situ phytoplankton productivity to the sediment signal is less here than further down the estuary towards the inner-shelf area. A similar phenomenon has been reported from other estuaries, e.g. the Gironde estuary, western Europe (Fontugne and Jouanneau, 1987) and the Yangtze River estuary, China (Wu et al., 2007). 4.4. Influences on the bulk organic δ13C and C/N 4.4.1 Anthropogenic influences It has been suggested that anthropogenic influence from the Pearl River delta, such as the agricultural products, human wastes and industrial inputs, has become an important factor that changes the organic carbon isotopic signature of the surface sediments in the estuary (Jia and Peng, 2003; Owen and Lee, 2004). Our results show that agricultural C3 plants have marginally higher δ13C (–28.2±1.4‰) than general C3 plants (–29.9±1.3‰), as well as the agricultural C4 plants and general C4 grass (Fig. 3). Correspondingly, δ13C decreases from agricultural soil samples to riverbank and mangrove soil, and the forest soil has the lowest δ13C of all (Fig. 4). C/N increases through these soil types, with the forest soil having the highest C/N. This increase in δ13C and decrease in C/N found in agricultural soil might be due to the fertilization process (O’Leary, 1985). Sediments deposited during recent decades tend to have elevated δ13C, which appear to coincide with rapid urbanisation, industrialisation and reclamation in this area (Owen and Lee, 2004; Hu et al., 2008). For example, well-nourished plants show higher δ13C than plants deficient in nitrogen and/or potassium (less fertilized) (O’Leary, 1985). As a relatively densely populated area, sewage could be a potentially important contributor to the sediment organic matter in the Pearl River estuary. Sediments from Victoria Bay, Hong Kong, were significantly contaminated by sewage (Hsieh, 2006). The δ13C of these sediments varies from −28.59 to −22.60‰ and C/N from 10.71 to 14.73 (Hsieh, 2006), which must include the influence of sewage. Here we have undertaken - 12 - biomarker analysis of the Pearl River surface samples (David Strong, pers. comm.) and show the lack of coprostanol and other 5-beta stanols indicating that sewage is an insignificant contributor to the organic carbon in these sediments. The main organic pollutants found in the estuarine area are the polycyclic aromatic hydrocarbons (PAHs) and polychlorinated biphenyles (PCBs) and organochlorinate pesticides (Kang et al., 2000). Concentrations of the total PAHs, PCBs and organochlorinate pesticides in surface sediments from the river to the estuarine area are 1 167 – 21 329 µg g-1, 10.2-485.5 µg g-1 and 5.8-1 658 µg g-1 respectively (Kang et al., 2000). When compared to the TOC values from surface samples analysed here of 0.5-2% TOC (i.e. 5 000-20 000 mg kg-1) the concentration of these pollutants is actually very low. Sediments with the highest concentration of these organic pollutants are from areas near Guangzhou and/or Maucau according to Kang et al. (2000). For example, concentration of organochlorinate pesticides in sediments from areas investigated by this study is usually between 5 and 70 µg g-1 (Kang et al., 2000). Overall we consider that the contribution of organic carbon from these pollutants is insignificant. A possibly more important contributor that might influence δ13C value of the sediment is crude oil. The light oil from the Pearl River estuary is characterized by low density (less than 0.83 g cm-3), high contents of saturated hydrocarbons (71%-93%) and no asphaltenes (Guo and He, 2006). δ13C of the crude oil from Pearl River estuary is between −27.09‰ and –26.48‰ (Guo and He, 2006), slight heavier than that of C3 plants. However, according to biomarker results (David Strong, pers. comm.), the total amount of lipids from crude oil in these samples ranges from 100 to 500 µg/g, which compared to the average TOC values of 0.5-2% (i.e. 5-20 mg/g, Table 5), indicates a maximum concentration of 10% (0.5% - 10%). Furthermore, biomarker results also suggested that the apolar freactions found in the crude oil would most likely already include the PCBs. These data indicate that sedimentary organic carbon contributors other than plants, planktons and algae, such as human waste, organic pollutants and crude oil, are less important. The maximum concentration of organic carbon from these anthropogenic sources is less than 10%. Thus, in spite of the influences of organic carbon from other sources, bulk organic δ13C and C/N is still a good indicator for the source of estuarine sediment at the broadest scale from terrestrial, lowest deltaic or marine areas. 4.4.2 Degradation Change in bulk sediment organic δ13C and C/N over time due to decomposition is an important consideration in coastal palaeoenvironmental research (e.g. Wilson et al., 2005; Lamb et al., 2006). For example, in the coastal wetlands of Louisiana, USA, sediment δ13C shifts between −0.5‰ and −3.3‰ from the surface vegetation (Chmura et al., 1987). Changes in C/N can also occur during decomposition, particularly in the early stages (Valiela et al., 1985). Lamb et al. (2006) reviewed different studies and concluded that in inter-tidal and supra-tidal sediments, this may result in much lower surface sediment C/N compared with the overlying vegetation, whereas in sub-tidal sediments, C/N is commonly slightly higher than in the suspended sediment phase. The influence of degradation varies across the Pearl River delta area and estuary. In the Pearl River delta, results show that soil organic matter generally has higher average δ13C but lower C/N (Table 2) than that of overlying vegetation (Table 1). For example, - 13 - average δ13C of mangrove soil is 3.1‰ higher than that of overlying mangrove plants, and average C/N of mangrove soil is 12.4 compared to 31.0 for that of mangrove plants. Degradation effect might be an important reason for differences in δ13C and C/N between SOM and overlying plants. However, SOM receives organic carbon from all overlying vegetation which is a mixture of both C3 and C4 types. Thus differences of δ13C between the mixed type of organic matter (SOM) and individual plants should also be significant. In the Pearl River estuary, sedimentary organic matter is mainly derived from riverine and marine areas (Zong et al., 2006). Much of the terrigenous component of riverine organic matter may have already been extensively degraded on land or further upstream and should be relatively resistant to further degradation (Hedge and Keil, 1995). Degradation of in situ POC might become significant for surface sediment δ13C. Results show that POC from both seasons exhibit much lower C/N ratios than surface sediments (Table 3, 5). The contrast is particularly obvious in fluvial areas (e.g. G2, G4), and becomes more similar in marine area (e.g. G6). Similar phenomenon in C/N ratio has been reported by Cifuentes (1991) from the Delaware Estuary, southeast USA. Cifuentes (1991) has also reported differences in δ13C values between the two phases. In the upper estuary, δ13C of POC (−28.9‰) was lower than that of surface sediment (−26.3‰), whilst in the lower estuary, suspended sediment δ13C (−20.1‰) was higher than surface sediment δ13C (−23.5‰) (Cifuentes, 1991). However, changes in δ13C between POC and surface sediment in the Pearl River estuary are not so significant when POC from both summer and winter are taken into consideration. This suggests that differential decomposition of organic detritus may partially explain these observations especially in C/N, seasonality may be more important in the Pearl River estuary, and possibly in the Delaware Estuary, USA (Cifuentes, 1991). Degradation can have a potentially significant influence on both sediment δ13C and C/N. Early loss of labile material in vascular vegetation can lead to significant shifts in soil δ13C and C/N but is insufficient to prevent the distinction between sediments from C3 and C4 vegetated soil. The degradation of POC in estuarine areas suggests using either proxy in isolation might produce misleading results. It is important to combine δ13C and C/N when indicating sediment sources and environmental changes from coastal areas (Wilson et al., 2005; Zong et al., 2006). 5. Conclusions 1. In the Pearl River delta, while C3 plants have lower δ13C than C4 plants, –29.0±1.8‰ and –13.1±0.5‰ respectively, C/N of both C3 and C4 are widely variable and the overlap between groups is considerable. 2. δ13C and C/N for terrestrial soil organic matter are highly related to the source of organic matter. Soil samples from C3-plant-dominant areas, e.g. the forest, tend to have lower δ13C than those from C4-grass-dominant areas, e.g. the riverside area. Their C/N, however, are widely variable and significantly overlap. Thus, while the δ13C of soil can be used to indicate the predominant vegetation type, C/N cannot. 3. δ13C and C/N of POC show significant variability due to seasonal variation of freshwater flux resulting in changes the balance between the TOM and in situ phytoplankton in the POC. - 14 - 4. δ13C of surface sediment in the estuary increases from –25.0‰ at the freshwater end to –21.0‰ at the marine end, with C/N decreasing from 15.2 to 6.8, suggesting a weakening terrestrial/freshwater input and a strengthening marine contribution seaward. 5. Organic carbon from other sources might influence δ13C of surface sediments. Agricultural input might elevate δ13C of sedimentary organic matter due to enhanced fertilization process and, more significantly, by an increase in the proportion of C4 plants such as sugarcane grown in the catchment area. Organic carbon contributed from crude oil and other anthropogenic inputs such as PAHs, PCBs and organochrolinate pesticides, counts up to 10% of the TOC in the sediment according to biomarker results, which might also elevate organic δ13C of the sediment. 6. Degradation accounts for some of the shift in δ13C and C/N between soil and overlying vegetation, as well as between surface sediments and POC. However, the change is not significant enough to prevent application of δ13C and C/N as indicator for dominant overlying vegetation type and sediment source. 7. Based on the analysis of modern samples of organic matter from source to sink in the Pearl River catchment, delta and estuary, we conclude that it is possible to use δ13C and C/N of bulk organic matter to indicate vegetation type of the source area, sedimentary environment such as range of salinity, and dominant sources of estuarine sediment at the broadest scale from terrestrial, estuarine and marine environment. Acknowledgements This research is part of the PhD project sponsored by NERC/EPSRC (UK) through the Dorothy Hodgkin Postgraduate Award (to FY). This research is also supported by the University of Durham through a special research grant (to YZ), the NERC (UK) Radiocarbon Laboratory Steering Committee (1150.1005) (to YZ) and the NERC Isotope Geosciences Facilities Steering Committee through the organic isotope analyses awarded (IP/883/1105) (to YZ). We also acknowledge support from the QRA (Quaternary Research Association), the DGGA (Durham Geography Graduates Association), University College of Durham University and the BSRG (British Sediment Research Group) for grants awarded to FY to complete fieldwork and all laboratory visits. This research was also supported by the Research Grants Council of the Hong Kong SAR through research grants HKU7058/06P and HKU7052/08P (to W.W.-S. Yim). The authors also thank the director of the Environmental Protection Department, Hong Kong SRA for the collection of surface sediment samples and water salinity from the Hong Kong area. - 15 - References Austin, A.T., Vitousek, P.M., 2005. Nutrient dynamics on a precipitation gradient in Hawai i. Oecologia 113, 519–529. Bender, M.M., 1971. Variations in the 13C/12C ratios of plants in relation to the pathway of photosynthetic carbon dioxide fixation. Phytochemistry 10(6), 1239-1244. Bianchi, T.S., Wysocki, L.A., Stewart, M., Filley, T.R., McKee, B.A., 2007. Temporal variability in terrestrially-derived sources of particulate organic carbon in the lower Mississppi River and its upper tributaries. Geochimica et Cosmochimica Acta 71, 4425-4437. Bird, M.I., Robinson, R.A.J., Oo, N.W., Aye, M.M., Lu, X.X., Higgitt, D.L., Swe, A., Tun, T., Win, S.L., Aye, K.S., Win, K.M.M., Hoey, T.B., 2008. A preliminary estimate of organic carbon transport by the Ayeyarwady (Irrawaddy) and Thanlwin (Salween) Rivers of Myanmar. Quaternary International 186(1), 113-122. Cao, C., Wang, W., Liu, L., Shen, S., Summons, R.E., 2008. Two episodes of 13Cdepletion in organic carbon in the latest Permian: Evidence from the terrestrial sequences in northern Xinjiang, China. Earth and Planetary Science Letters 270(3-4), 251-257. Callahan, J., Dai, M., Chen, R.-F., Li, X., Lu, Z., Huang, W., 2004. Distribution of dissolved organic matter in the Pearl River Estuary, China. Marine Chemistry 89, 211 – 224. Chen, J., Jin, H., Yin, K., Li, Y., 2003. Variation of reactivity of particulate and sedimentary organic matter along the Zhujiang River Estuary. Acta Oceanologica Sinica 22, 557– 568. Chen, J., Li, Y., Yin, K., Jin, H., 2004. Amino acids in the Pearl River Estuary and the adjacent waters: origins, transformation and degradation. Continental Shelf Research 24, 1877–1894. Chivas, A.R., Garcia, A., van der Kaars, S., Couapel, M.J.J., Holt, S., Reeves, J.M., Wheeler, D.J., Switzer, A.D., Murray-Wallace, C.V., Banerjee, D., Price, D.M., Wang, S.X., Pearson, G., Edger, N.T., Beaufort, L., De Deckker, P., Lawson, E., Cecil, C.B., 2001. Sea level and environmental changes since the last interglacial in the Gulf of Carpentaria, Australia: an overview. Quaternary International 83-85, 19-46. Chmura, G.L., Aharon, P., Socki, R.A., Abernethy, R., 1987. An inventory of 13C abundances in coastal wetlands of Louisiana, USA: vegetation and sediments. Oecologia 74, 264–271. Cifuentes, L.A., 1991. Spatial and temporal variations in terrestrially-derived organic matter from sediments of the Delaware Estuary. Estuaries 14, 414– 429. Countway, R.E., Canuel, E.A., Dickhut, R.M., 2007. Sources of particulate organic matter in surface waters of the York River, VA estuary. Organic Geochemistry 38(3), 365-379. Craig, H., 1953. The geochemistry of the stable carbon isotopes. Geochimica et Cosmochimica Acta 3(2-3), 53-92. Dai, M., Martin, J., Hong, H., Zhang, Z., 2000. Preliminary study on the dissolved and colloidal organic carbon in the Zhujiang River Estuary. Chinese Journal of Oceanology and Limnology 18, 265–273. - 16 - Driese, S.G., Li, Z.-H., Horn, S.P., 2005. Late Pleistocene and Holocene climate and geomorphic histories as interpreted from a 23,000 14C yr B.P. paleosol and floodplain soils, southeastern West Virginia, USA. Quaternary Research 63(2), 136-149. Driese, S.G., Li, Z.-H., McKay, L.D., 2008. Evidence for multiple, episodic, midHolocene Hypsithermal recorded in two soil profiles along an alluvial floodplain catena, southeastern Tennessee, USA. Quaternary Research 69(2), 276-291. Emerson, S, Hedges, J.I., 1988. Processes controlling the organic carbon content of open ocean sediments. Paleoceanography 3, 621–634. Fan, M., Dettman, D.L., Song, C., Fang, X., Garzione, C.N., 2007. Climatic variation in the Linxia basin, NE Tibetan Plateau, from 13.1 to 4.3 Ma: The stable isotope record. Palaeogeography, Palaeoclimatology, Palaeoecology 247(3-4), 313-328. Fontugne, M.R., Jouanneau, J.-M., 1987. Modulation of the particulate organic carbon flux to the ocean by a macrotidal estuary: Evidence from measurements of carbon isotopes in organic matter from the Gironde system. Estuarine, Coastal Shelf Science 24(3), 377-387. Fry, B., Sherr, E.B., 1984. δ13C measurements as indicators of carbon flow in marine and freshwater ecosystems. Contributions in Marine Science 27, 13–47. Gaye, B., Fahl, K., Kodina, L.A., Niko, L.N., Nagel, B., Unger, D., Gebhardt, A.C., 2007. Particulate matter fluxes in the southern and central Kara Sea compared to sediments: Bulk fluxes, amino acids, stable carbon and nitrogen isotopes, sterols and fatty acids. Continental Shelf Research 27(20), 2570-2594. Goñi, M.A., Ruttenberg, K.C., Eglinton, T.I., 1997. Sources and contribution of terrigenous organic carbon to surface sediments in the Gulf ofMexico. Nature 389, 275-278. Guo, X., He, S., 2006. Geochemical Characteristics and Origin of the Light Crude Oils in Panyu Lower Uplift, Pearl River Mouth Basin. Geological Science and Technology Information 25(5), 63-68 (in Chinese, with English abstract). Harmelin-Vivien, M., Loizeau, V., Mellon, C., Beker, B., Arlhac, D., Bodiguel, X., Ferraton, F., Hermand, R., Philippon, X., Salen-Picard, C., 2008. Comparison of C and N stable isotope ratios between surface particulate organic matter and microphytoplankton in the Gulf of Lions (NW Mediterranean). Continental Shelf Research 28, 1911-1919. de Haas, H., van Weering, T.C.E., de Stigter, H., 2002. Organic carbon in shelf seas: sinks or sources, processes, and products. Continental Shelf Research 22, 691–717. Hatch, M.D., Slack, C.R., 1970. Photosynthetic CO2-fixation pathways. Annual Review of Plant Physiolology 21, 141-162. Hedges, J.I., Keil, R.G., 1995. Sedimentary organic matter preservation: an assessment and speculative synthesis. Marine Chemistry 49, 81–115. Hedges, J.I., Parker, P.L., 1976. Land-derived organic matter in surface sediments from the Gulf of Mexico. Geochimica et Cosmochimica Acta 40, 1019–1029. Hsieh, M., 2006. Geochemical characterization of organic matter in Victoria Harbour sediments, Hong Kong. PhD thesis, University of Oklahoma, Japan. Huang, L., Jian, W., Song, X., Huang, X., Liu, S., Qian, P., Yin, K., Wu, M., 2004. Species diversity and distribution for phytoplankton of the Pearl River estuary during rainy and dry seasons. Marine Pollution Bulletin 49, 588–596. - 17 - Huang, X.P., Huang, L.M., Yue, W.Z., 2003. The characteristic of nutrients and eutrophication in the Pearl River estuary, South China. Marine Pollution Bulletin 47, 30– 36. Hu, J., Peng, P., Jia, G., Mai, B., Zhang, G., 2006. Distribution and sources of organic carbon, nitrogen and their isotopes in sediments of the subtropical Pearl River estuary and adjacent shelf, Southern China. Marine Chemistry 98, 274– 285 Hu, J., Zhang, G., Li, K., Peng, P., Chivas, A.R., 2008. Increased eutrophication offshore Hong Kong, China during the past 75 years: Evidence from high-resolution sedimentary records. Marine Chemistry 110(1-2), 7-17. Jia, G., Peng, P., 2003. Temporal and spatial variations in signatures of sedimented organic matter in Lingding Bay (Pearl estuary), southern China. Marine Chemistry 82, 47– 54. Kang, Y., Mai, B., Huang, X., Zhang, G., Sheng, G., Fu, J., 2000. Primary study on status of organic pollution in surface sediments of the Pearl River Delta. Acta Science Circumstantiae 20, 164-170 (in Chinese, with English abstract). Lamb, A.L., Wilson, G.P., Leng, M.J., 2006. A review of coastal palaeoclimate and relative sea-level reconstructions using δ13C and C/N ratios in organic material. EarthScience Review 75, 29-57. Li, P., Qiao, P., Zheng, H., Fang, G., Huang, G., 1990. The environmental evolution of the Pearl River Delta in the last 10,000 years (in Chinese). China Ocean Press, Beijing. Liu, W., An, Z., Zhou, W., Head, M.J., Cai, D., 2003. Carbon isotope and C/N ratios of suspended matter in rivers: an indicator of seasonal change in C4/C3 vegetation. Applied Geochemistry 18, 1241-1249. Liu, K.-K., Kao, S.-J., Hu, H., Chou, W., Hung, G., Tseng, C., 2007. Carbon isotopic composition of suspended and sinking particulate organic matter in the northern South China Sea--From production to deposition. Deep Sea Research (Part II, Topical Studies in Oceanography) 54(14-15), 1504-1527. Mackie, E.A.V., Leng, M.J., Lloyd, J.M., Arrowsmith, C., 2005. Bulk organic δ13C and C/N ratios as palaeosalinity indicators within a Scottish isolation basin. Journal of Quaternary Science 20, 301-408. Mackie, E.A.V., Lloyd, J.M., Leng, M.J., Bentley, M.J., Arrowsmith, C., 2007. Assessment of δ13C and C/N ratios in bulk organic matter as palaeosalinit indicators in Holocene and Lateglacial isolation basin sediments, northwest Scotland. Journal of Quaternary Science 22(6), 579-591. Meyers, P.A., 1997. Organic geochemical proxies of paleoceanographic, paleolimnologic, and paleoclimatic processes. Organic Geochemistry 27, 213– 250. Middelburg, J.J., Nieuwenhuize, J., 1998. Carbon and nitrogen stable isotopes in suspended matter and sediments from the Schelde Estuary. Marine Chemistry 60, 217225. Middelburg, J.J., Herman, P.M.J., 2007. Organic matter processing in tidal estuaries. Marine Chemistry 106(1-2), 127-147. Morton, B., Wu, S.S., 1975. The hydrology of the coastal waters of Hong Kong. Environmental Research 10,319–347. O’Leary, M.H., 1985. Carbon isotope fractionation in plants. Phytochemistry 20, 553-567. Owen, R.B., Lee, R., 2004. Human impacts on organic matter sedimentation in a proximal shelf setting, Hong Kong. Continental Shelf Research 24, 583–602 - 18 - Park, R., Epstein, S., 1960. Carbon isotope fractionation during photosynthesis. Geochimica et Cosmochimica Acta 21(1-2), 110-126. Ramaswamy, V., Gaye, B., Shirodkar, P.V., Chivas, A.R., Wheeler, D., Thwin, S., 2008. Distribution and sources of organic carbon, nitrogen and their isotopic signatures in sediments from the Ayeyarwady (Irrawaddy) continental shelf, northern Andaman Sea. Marine Chemistry 111 (3-4), 137-150. Saia, S.E.M.G., Pessenda, L.C.R., Gouveiaa, S.E.M., Aravena, R., Bendassolli, J.A., 2008. Last glacial maximum (LGM) vegetation changes in the Atlantic Forest, southeastern Brazil. Quaternary International 184(1), 195-201. Schlunz, B., Schneider, R.R., Muller, P.J., Showers, W.J., Wefer, G., 1999. Terrestrial organic carbon accumulation on the Amazon deep sea fan during the last glacial sea level low stand. Chemical Geology 159, 263–281. Schultz, D. and Calder, J.A., 1976. Organic carbon 13C/12C variations in estuarine sediments. Geochimica et Cosmochimica Acta 40, 381–385. Tan, Y., Hiang, L., Chen, Q., Huang, X., 2004. Seasonal variation in zooplankton composition and grazing impact on phytoplankton standingstock in the Pearl River Estuary, China. Continental Shelf Research 24, 1949–1968. Tesi, E., Miserocchi, S., Goñi, M.A., Langone, L., Boldrin, A., Turchetto, M., 2007. Organic matter origin and distribution in suspended particulate materials and surficial sediments from the western Adriatic Sea (Italy). Estuarine. Coastal Shelf Science 73(3-4), 431-446. Valiela, I., Teal, J.M., Allen, S.D., 1985. Decomposition in salt marsh ecosystems: the phases and major factors affecting disappearance of above-ground organic matter. Journal of Experimental Marine Biology and Ecology 89, 29–54. Wai, O.W.H., Wang, C.H., Li, Y.S., Li, X.D., 2004. The formation mechanisms of turbidity maximum in the Pearl River estuary, China. Marine Pollution Bulletin 48, 441–448. Wilson, G.W., Lamb, A.L., Leng, M.J., Gonzalez, S., Huddart, D., 2005. Variability of organic δ13C and C/N in the Mersey Estuary, UK and its implications for sea-level reconstruction studies. Estuarine, Coastal Shelf Science 64, 685– 698. Winkler M. G. and Wang P. K., 1993. The late Quaternary vegetation and climate of China, In: Wright W. H. E., Jr., Kutzbach J. E., Webb III T., Ruddiman W. F., StreetPerrott F. A. and Bartlein P. J. (Eds.), Global Climates Since The Last Glacial Maximum. University of Minnesoda Press, Minneapolis, MN, 221–264. Wu, Y., Zhang, J., Liu, S.M., Zhang, Z.F., Yao, Q.Z., Hong, G.H., Cooper, L., 2007. Sources and distribution of carbon in the Yangtze River (Changjiang) system. Estuarine Coastal Shelf Science 71, 13–25. Xu, J.L., Li, Y.X., Can, F.X., Chen, Q.D., 1985. Evolution of channels and shoals in Lingding Sea Pearl River Estuary. Ocean Press, Beijing (in Chinese). Yin, K., Zhang, J., Qian, P., Jian, W., Huang, L., Chen, J., Wu, M.C.S., 2004. Effect of wind events on phytoplankton blooms in the Pearl River estuary during summer. Continental Shelf Research 24, 1909–1923 Zhang, J., Wu, Y., Jennerjahn, T.C., Ittekkot, V., He, Q., 2007. Distribution of organic matter in the Changjiang (Yangtze River) Estuary and their stable carbon and nitrogen isotopic ratios: Implications for source discrimination and sedimentary dynamics. Marine Chemistry 106(1-2), 111-126. - 19 - Zhang, S., Lu, X., Higgitt, D.L., Chen, C.A., Han, J., Sun, H., 2008. Recent changes of water discharge and sediment load in the Zhujiang (Pearl River) Basin, China. Global and Planetary Change 60, 365–380. Zong, Y., Lloyd, J.M., Leng, M.J., Yim, W.W.-S., Huang, G., 2006. Reconstruction of Holocene monsoon history from the Pearl River estuary, southern China, using diatoms, carbon isotope ratios. The Holocene 16, 1-13. Zong, Y., Huang, G., Switzer, A.D., Yu, F., Yim, W.W.-S., 2009. An evolutionary model for the Holocene formation of the Pearl River delta, China. The Holocene 19, 129-142. - 20 - Figure captions Fig. 1 Study area, showing the Pearl River delta and estuary in the East Asian area (a) and sampling sites (b). There is no surface sediment from site PE43 and no winter POC from site PE76 and 77. ‘PE’ stands for the ‘Pearl River estuary’. Fig. 2 Clustering groups for the estuarine surface sediment samples and POC samples identified by winter salinity and sand concentration. Groups G1-G3 are from freshwater areas; groups G4-G7 are from brackish areas and G8-G9 are from marine areas. Fig. 3 Bi-plot of the δ13C and C/N of C3 and C4 plants. a: individual values for each plant with the average value of C3 and C4 plants; b: average values of different types of plants within the C3 and C4 groups (error bars are the standard deviation). Fig. 4 Bi-plot of the δ13C and C/N of terrestrial soil samples. a: individual values for each soil sample; b: average values of different types of soil with stand deviation. Fig. 5 Bi-plot of seasonal δ13C and C/N of the POC. Fig. 6 A summarized bi-plot of seasonal δ13C and C/N (a: winter and b: summer) and biplot of seasonal δ13C and water salinity (c: winter and d: summer) of the POC for each group indentified by cluster analysis. Fig. 7 Comparison δ13C and C/N values between the POC on 70 µm net and summer POC on filter paper. Fig. 8 Bi-plot of the δ13C and C/N of surface sediment groups. a: scattering value of the individual sample; b:average δ13C and C/N values of the seven groups with standard deviation shown. - 21 - Tables General terrestrial C3 plants Table 1 Results of the plants samples and their sampling sites. Agricultural C3 plants Ave. Mangroves C3 plants Ave. Ave. General C4 plants Ave. Agricult ural C4 plants C4 plants Ave. Ave. Ave. δ13C (‰) -28.5 -29.9 -29.3 -28.1 -30.3 -28.1 -31.0 -29.9 -28.3 -29.6 -29.6 -31.4 -30.2 -30.7 -28.9 -29.5 -28.7 -28.7 -28.3 -30.6 -30.9 -30.2 -29.9 -32.6 -31.7 -30.4 -31.6 -30.0 -31.8 -28.0 -28.3 -28.4 -32.1 -29.9 ±1.3 -28.2 -30.2 -28.7 -28.6 -28.9 -27.3 -25.8 -28.2 ±1.4 -28.9 -27.9 -28.1 -28.5 -25.0 -25.0 -24.0 -28.4 -25.6 -28.3 -28.1 -27.4 -27.1 ±1.7 -29.0 ±1.8 TOC (%) 41.6 46.7 42.0 43.3 45.3 40.1 45.3 40.9 41.1 42.3 38.6 45.1 44.3 36.6 37.0 39.2 39.6 48.1 44.9 42.2 44.4 47.2 38.0 43.2 39.2 40.8 42.3 41.1 40.3 45.6 48.1 35.2 48.0 42.3 ±3.5 42.0 35.7 38.0 40.0 39.4 43.2 44.1 40.3 ±3.0 45.3 46.6 49.1 45.8 43.6 42.2 40.7 46.7 41.1 45.4 44.2 45.1 44.6 ±2.4 42.6 ±3.4 TN (%) 3.1 3.7 2.3 5.9 3.9 1.9 1.8 2.9 4.2 2.0 2.0 1.4 2.1 1.9 3.5 2.4 3.8 0.8 1.4 1.9 2.1 1.6 1.9 2.1 1.7 1.9 3.1 1.2 0.9 2.1 1.6 2.4 2.0 2.4 ±1.1 2.8 2.3 3.4 3.3 3.5 2.4 3.5 3.0 ±0.5 2.0 1.4 0.9 1.3 0.9 2.2 3.0 1.3 1.8 1.7 1.0 2.3 1.7 ±0.6 2.3 ±1.0 C/N 13.4 12.5 18.1 7.4 11.5 21.4 25.4 14.0 9.8 21.0 19.1 31.2 20.8 19.2 10.7 16.3 10.4 61.8 31.1 22.2 20.9 30.3 20.1 20.5 23.5 21.7 13.5 33.3 43.1 21.6 30.2 14.6 24.2 21.7 ±10.7 15.1 15.3 11.1 12.2 11.1 18.3 12.6 13.7 ±2.7 22.5 32.8 53.2 34.5 46.3 19.1 13.4 36.6 22.4 27.2 45.2 19.4 31.0 ±12.5 22.7 ±11.6 Type C3 Plant C3 Plant C3 Plant C3 Plant C3 Plant C3 Plant C3 Plant C3 Plant C3 Plant C3 Plant C3 Plant C3 Plant C3 Plant C3 Plant C3 Plant C3 Plant C3 Plant C3 Plant Dizygoyheca elegantissima Pteris semipinnata C3 Plant C3 Plant C3 Plant C3 Plant C3 Plant C3 Plant C3 Plant C3 Plant C3 Plant C3 Plant C3 Plant C3 Plant C3 Plant Label B1 B2 B3 B4 B5 B6 B7 B8 B9 B10 B11 B12 B13 B14 B15 B16 B17 B18 B19 B20 B21 B22 B23 B24 B25 B26 B27 B28 B29 B31 B32 B35 B47 Site N1 N2 N2 N2 N3 N3 N3 N3 N4 N4 N4 N4 N4 N5 N5 N5 N5 N6 N6 N6 N6 N6 N6 N6 N6 N6 N6 N6 N6 E1 E1 E1 E1 Reeds Reeds Rice Rice Rice Banana Lotus F3 F5 F10 F11 F13 F12 F14 E5 E5 E5 E5 E5 E5 E5 Avicennia Kandelia obovata, Rhizophoraceae Kandelia obovata, Rhizophoraceae Kandelia obovato, Rhizophoraceae Bruguiera gymhorrhiza, Rhizophoraceae Acanthus ilicifolius, flowers Aegiceras corniculatum, Myrsinaceae Acanthus ilicifolius, Acanthaceae Acanthus ilicifolius, Acanthaceae Avicennia marina, Avicenniaceae Ficus microcarpa, Moraceae Ficus microcarpa, Moraceae B30 B36 B45 B42 B44 B37 B38 B40 B41 B43 B39 B46 E2 E3 E4 E4 E4 E3 E3 E3 E3 E4 E3 E4 -13.1 -13.2 -13.7 -13.7 -13.3 -12.3 -13.2 ±0.5 -12.9 -12.8 -12.5 -12.7 ±0.2 -13.1 ±0.5 35.5 39.8 40.0 41.2 38.8 39.7 39.2 ±1.9 41.6 42.4 41.4 41.8 ±0.5 40 ±2.0 4.3 1.2 1.7 1.7 1.5 2.8 2.2 ±1.2 1.0 1.6 1.6 1.4 ±0.3 1.9 ±1.0 8.2 33.1 23.9 23.8 26.1 14.2 21.5 ±8.9 40.3 25.9 25.6 30.6 ±8.4 24.6 ±9.4 C4 grass C4 grass C4 grass Panicum maximum Jacq C4 grass C4 grass F6 F1 F2 B33 B34 F4 E5 E5 E5 E1 E1 E5 sugarcane sugarcane sugarcane F7 F8 F9 E5 E5 E5 - 22 - Forest soil Table 2 Results of the soil samples and their sampling sites. Riverbank soil Ave. Mangrove soil Ave. Agricult ural soil Ave. Ave. δ13C (‰) -28.9 TOC (%) 2.7 TN (%) 0.2 C/N 15.4 Label A5 Site N4 -27.7 3.9 0.2 20.4 A9 N6 -28.3 ±0.8 3.3 ±0.9 0.2 ±0.0 17.9 ±3.6 -23.2 -22.9 -24.9 -23.6 -25.0 -23.6 -24.0 -25.6 -24.1 ±1.0 1.9 1.2 2.0 1.4 2.6 0.9 3.1 3.4 2.1 ±1.0 0.2 0.1 0.2 0.1 0.1 0.1 0.2 0.3 0.2 ±0.1 12.1 9.8 12.7 11.1 17.3 10.7 13.4 12.7 12.5 ±2.3 A1 A2 A3 A4 A6 A7 A8 G1 N1 N2 N3 N4 N4 N5 N5 E5 -25.6 -23.8 -24.7 -24.2 -26.1 -25.4 -19.3 -22.5 -25.8 -23.0 -23.8 -24.0 -24.0 ±1.9 2.7 1.1 1.8 2.4 1.8 1.1 4.4 0.9 2.4 2.5 0.7 1.5 1.9 ±1.0 0.3 0.1 0.2 0.2 0.1 0.1 0.3 0.0 0.1 0.2 0.1 0.1 0.2 ±0.1 9.7 8.6 9.3 11.4 12.9 11.0 15.7 19.3 18.8 10.4 10.7 10.8 12.4 ±3.6 A10 A11 A12 A13 A14 A15 A16 A17 A18 A19 A20 PE76 E3 E3 E3 E3 E3 E3 E3 E3 E4 E4 E4 E1 -21.2 -22.5 -21.5 -21.7 ±0.7 1.6 1.7 2.3 1.9 ±0.4 0.2 0.2 0.3 0.2 ±0.1 9.9 9.1 7.7 8.9 ±1.1 G2 G3 G4 E5 E5 E5 - 23 - Table 3 Results of the POC samples and water conditions of the sampling sites (to be continued). Gr. PE 41 δ13C-W (‰) C/N-W δ13C-S (‰) C/N-S Sal-W (‰) Sal-S (‰) PH-W PH-S TDS-W (g/l) TDS-S (g/l) DO-W (%) DO-S (%) Temp-W (ºC) Temp-S (ºC) -27.3 6.4 -25.0 8.3 0.9 0.1 7.7 7.5 1.2 0.1 98.5 79.3 17.4 28.2 PE 42 G2 6.6 -25.4 9.0 0.2 0.1 7.5 7.4 0.2 0.1 94.0 79.6 16.9 29.2 PE43 -27.8 6.9 -25.2 8.6 0.1 0.1 7.5 7.4 0.2 0.1 93.0 94.0 17.2 28.9 PE 44 -28.5 6.9 -25.2 8.5 0.1 0.1 7.6 7.4 0.2 0.1 93.3 88.6 17.1 28.8 PE 45 -28.3 6.5 -25.4 8.1 0.1 0.1 7.5 7.4 0.2 0.1 94.0 88.7 16.7 28.9 PE 46 -27.5 8.1 -24.8 9.0 0.1 0.1 7.5 7.4 0.2 0.1 96.5 87.8 16.6 28.8 PE 47 -26.2 6.5 -23.3 9.6 0.1 0.1 7.6 7.5 0.2 0.2 85.5 81.0 17.7 27.9 -27.6±0.8 6.8±0.6 -24.9±0.7 8.7±0.5 0.2±0.3 0.1±0.0 7.5±0.1 7.4±0.0 0.3±0.4 0.1±0.0 93.5±4.1 85.6±5.6 17.1±0.4 28.7±0.4 -23.0 10.0 0.8 0.1 7.6 7.6 Ave. PE 48 G4 27.8 -24.7 7.5 -22.9 9.9 0.5 0.1 7.6 7.5 0.6 0.2 87.5 84.8 17.5 28.0 PE 50 -26.0 7.6 -23.4 7.6 1.8 0.1 7.6 7.5 2.2 0.2 89.5 75.1 17.6 27.8 PE 51 -25.2 7.5 -24.3 11.3 1.5 0.1 7.6 7.6 1.8 0.2 89.0 76.3 17.7 27.8 PE 52 -26.0 7.5 -23.6 10.2 1.5 0.1 7.6 7.7 1.9 0.2 90.2 76.3 17.6 27.9 PE 53 -26.0 7.3 -24.1 10.1 4.3 0.1 7.7 7.6 5.0 0.2 101.9 72.5 17.2 28.1 PE 56 -24.7 7.2 -24.8 9.2 12.5 0.1 7.8 7.8 13.5 0.2 115.0 83.8 16.6 27.8 PE 54 -27.7 6.4 -25.6 6.9 2.3 0.1 7.7 7.6 2.8 0.2 96.6 73.8 17.6 27.7 -25.8±1.0 7.3±0.4 -24.0±0.9 9.4±1.5 3.1±4.0 0.1±0.0 7.7±0.1 7.6±0.1 4.0±4.4 0.2±0.0 95.7±9.9 79.3±6.8 17.4±0.4 27.8±0.1 4.8 0.1 PE 76 Ave. 92.1 PE 49 Ave. G3 0.2 PE 55 -26.4 6.8 -24.9 8.8 6.3 0.1 7.8 7.7 7.2 0.2 106.6 84.5 17.1 27.6 PE 59 -23.7 6.5 -25.9 8.1 23.1 2.9 8.0 7.9 23.6 3.8 112.0 86.5 18.3 28.5 PE 60 -24.0 7.7 -26.1 8.8 26.9 5.9 8.0 7.8 27.1 6.9 109.9 79.7 18.5 28.3 PE 61 -23.9 7.7 -24.7 9.8 22.1 4.0 7.9 7.7 22.8 4.6 108.7 77.2 18.6 28.4 -24.5±1.3 7.2±0.6 -25.4±0.7 8.9±0.7 16.6±10.3 2.6±2.5 7.9±0.1 7.7±0.1 20.2±8.9 3.9±2.8 109.3±2.3 82.0±4.3 18.1±0.7 28.2±0.4 S: summer; W: winter; ‘Gr.’ shows different clustering groups of the POC samples. 24 Table 3 Results of the POC samples and water conditions of the sampling sites (continued). Gr. G5 PE 69 PE 70 PE 71 PE 72 PE 73 PE 64 PE 57 PE 58 PE 62 PE 63 PE 75 δ13C-W (‰) C/N-W δ13C-S (‰) C/N-S Sal-W (‰) Sal-S (‰) PH-W PH-S TDS-W (g/l) TDS-S (g/l) DO-W (%) DO-S (%) Temp-W (ºC) Temp-S (ºC) -22.3 -21.6 -22.5 -21.8 -21.7 -23.9 -24.0 -24.8 -25.2 -23.8 -25.0 7.5 8.3 6.8 8.6 8.7 8.4 9.9 6.1 7.3 8.9 8.9 -24.4 -25.7 -26.1 -25.6 -25.7 -23.3 -23.0 -23.1 -23.0 -23.3 -22.9 8.8 10.6 31.5 33.6 33.9 32.8 32.3 18.6 11.8 13.1 11.6 13.4 14.1 3.4 6.6 5.8 3.6 5.7 0.2 0.1 1.0 0.2 0.1 0.1 8.1 8.15 8.1 8.1 8.2 8.0 7.9 7.9 7.9 7.9 7.9 8.2 8.1 8.1 8.3 8.2 8.0 7.9 7.9 8.1 8.0 8.0 31.3 33.2 33.5 32.5 32.0 19.5 12.8 14.1 12.6 14.0 14.8 4.7 7.6 6.7 4.3 7.7 0.2 0.2 1.4 0.2 0.2 0.2 113.2 111.6 110.7 116.8 113.1 113.6 116.0 115.7 104.0 114.3 113.0 80.4 79.6 81.2 86.4 93.7 97.0 92.3 89.2 86.1 100.1 102.8 18.1 18.6 18.5 18.1 18.1 17.0 16.5 17.1 17.4 16.6 17.5 28.1 28.2 28.2 28.2 28.2 28.8 27.9 28.1 27.9 28.6 28.2 14.8 7.0 10.1 9.8 11.4 9.9 8.0 11.3 PE 77 PE 65 PE 66 PE 67 PE 68 PE 74 PE 78 PE 79 PE 80 PE 81 Ave. G6 Ave. PE 82 PE 83 PE 84 PE 85 PE 86 PE 87 PE 88 PE 89 PE 90 PE 91 PE 92 -23.6 -24.5 -24.9 -25.1 -23.9 -23.4 -23.2 -22.9 -24.3 7.3 8.2 6.5 6.8 6.8 7.1 6.4 6.9 7.1 -24.1 -24.6 -24.7 -24.6 -25.1 8.7 8.1 9.6 9.4 8.2 22.2 23.9 22.3 25.7 24.3 25.4 25.5 16.9 18.1 0.8 5.5 2.7 4.9 2.0 13.5 15.2 18.2 15.8 8.1 8.0 8.0 8.1 8.1 7.9 8.0 7.9 7.8 8.0 7.9 8.3 8.2 8.0 22.8 24.4 22.9 26.7 24.8 25.8 25.9 27.2 28.7 1.1 6.5 3.3 5.7 2.3 113.0 115.0 117.0 117.2 115.4 91.6 95.8 93.6 94.7 92.7 90.2 88.1 85.1 88.7 17.1 16.9 16.9 17.6 17.6 17.6 17.9 18.2 18.0 29.1 29.5 29.1 28.2 28.3 -23.6±1.2 7.6±1.0 -24.3±1.1 9.5±1.2 22.2±7.6 5.4±5.7 8.0±0.1 8.1±0.1 24.0±7.2 3.3±2.9 109.8±8.6 89.6±6.7 17.6±0.6 28.4±0.5 -23.0 -22.6 -23.3 -21.9 -22.6 -21.7 -22.5 -22.6 -22.2 -21.8 -22.3 7.2 6.9 5.9 5.7 6.4 6.2 6.1 6.9 5.9 6.1 6.3 32.9 31.9 30.9 35.0 31.3 34.7 30.8 32.5 34.3 35.1 34.8 18.6 22.0 20.3 24.0 25.4 27.5 20.0 25.0 26.2 28.2 28.4 7.9 8.1 8.1 7.9 8.1 8.2 8.1 8.0 8.2 8.2 8.0 32.5 31.7 30.6 34.4 31.1 34.1 30.7 32.2 33.8 34.5 34.2 96.4 96.0 99.0 101.0 98.5 97.0 94.1 94.8 96.3 97.4 93.5 18.8 19.0 19.2 19.8 18.8 19.4 19.0 18.9 19.3 19.7 19.4 -22.4±0.5 6.3±0.5 33.1±1.7 24.1±3.5 8.1±0.1 32.7±1.6 96.7±2.2 19.2±0.3 S: summer; W: winter; ‘Gr.’ shows different clustering groups of the POC samples. 25 26 Table 4 Results of the POC samples on the70 µm net (summer) and on the filter paper Summer POC (70 µm net) PN50 PN51 PN52 PN53 PN56 PN57 PN63 PN64 PN72 PN65 PN67 PN68 PN71 PN73 Ave. SD δ13C C/N -25.9 -25.8 -24.8 -24.4 -25.4 -25.1 -25.1 -25.1 -24.2 -27.2 -26.2 -27.0 -27.2 18.4 14.6 12.1 12.5 9.7 12.7 13.6 13.8 15.3 5.7 4.6 6.5 5.0 5.5 +10.7 +4.5 -25.6 +1.0 Summer POC (filter paper) PS50 PS51 PS52 PS53 PS56 PS57 PS63 PS64 PS72 PS65 PS67 PS68 PS71 PS73 δ13C C/N -23.4 -24.3 -23.6 -24.1 -24.8 -23.0 -23.3 -23.3 -25.6 -24.1 -24.7 -24.6 -26.1 -25.7 -24.3 +1.0 7.6 11.3 10.2 10.1 9.2 9.8 8.0 10.1 8.7 9.6 9.4 +9.5 +1.1 Winter POC (filter paper) δ13C C/N PW50 PW51 PW52 PW53 PW56 PW57 PW63 PW64 PW72 PW65 PW67 PW68 PW71 PW73 -26.0 -25.2 -26.0 -26.0 -24.7 -24.0 -23.8 -23.9 -21.8 -23.6 -24.9 -25.1 -22.5 -21.7 -24.2 +1.5 7.6 7.5 7.5 7.3 7.2 9.9 8.9 8.4 8.6 7.3 6.5 6.8 6.8 8.7 +7.8 +1.0 PS: summer plankton samples on filter paper; PW: winter plankton samples on filter paper; PN: summer plankton samples on a 70µm net; S.D.: standard deviation. - 27 - Table 5 Results of the surface sediment and environmental variables of the sampling sites (to be continued) Gr.(n) Sample ID Long (ºE) Lat(ºN) δ13C (‰) C/N TOC (%) TN(%) PE 17 PE 18 G1(4) PE 19 PE 20 Ave. PE 16 PE 15 PE 14 PE 10 PE 09 PE 11 G2(14) PE 12 PE 13 PE 41 PE 42 PE 44 PE 45 PE 46 PE 47 Ave. PE 48 PE 49 PE 50 PE 51 G3(8) PE 52 PE 53 PE 56 PE 54 Ave. PE 76 PE 01 PE 55 PE 08 PE 03 G4(11) PE 05 PE 06 PE 07 PE 59 PE 60 PE 61 Ave. -26.6 -23.3 -25.1 -25.0 -25.0±1.3 -23.7 -25.4 -22.7 -22.8 -23.6 -23.4 -22.7 -24.5 -24.8 -23.4 3.79 0.91 1.88 2.13 2.2±1.2 1.02 1.28 0.74 1.14 0.99 1.50 1.11 1.30 1.39 0.79 0.28 0.22 1.37 0.45 1.0±0.4 0.98 1.03 1.41 0.75 0.52 1.14 0.77 1.54 1.0±0.3 1.49 1.02 1.40 1.51 1.17 1.32 1.27 1.13 1.34 0.66 0.38 1.2±0.4 113.57 113.55 113.52 113.48 22.95 23.00 23.05 23.10 113.58 113.62 113.65 113.64 113.67 113.58 113.54 113.49 113.53 113.44 113.37 113.33 113.47 113.36 22.88 22.82 22.77 22.68 22.63 22.76 22.78 22.80 22.77 22.80 22.88 22.88 22.86 22.78 113.39 113.46 113.52 113.54 113.57 113.59 113.55 113.62 22.75 22.72 22.68 22.66 22.64 22.61 22.49 22.57 113.65 113.64 113.63 113.72 113.74 113.83 113.80 113.76 113.72 113.75 113.72 22.44 22.58 22.53 22.61 22.45 22.54 22.52 22.56 22.47 22.50 22.55 20.1 12.6 13.8 14.4 15.2±3.3 13.4 14.6 11.4 11.4 13.0 13.4 13.9 16.3 15.9 11.7 10.5 10.2 -23.0 12.8 -26.8 12.9 -23.9±1.2 13.0±1.8 -22.0 10.7 -21.9 9.2 -26.7 11.4 -22.6 10.9 -23.8 13.4 -22.0 10.7 -22.2 10.0 -22.9 12.3 -23.0±1.6 11.1±1.3 -24.0 10.8 -24.2 17.5 -23.9 17.1 -24.1 12.7 -23.1 11.5 -23.3 12.3 -23.5 14.6 -23.5 17.7 -23.4 10.5 -24.2 14.9 -27.0 15.1 -24.0±1.1 14.1±2.7 0.11 0.07 0.14 0.15 0.1±0.0 0.08 0.09 0.07 0.10 0.08 0.11 0.08 0.08 0.09 0.07 0.03 0.02 0.11 0.04 0.1±0.0 0.09 0.11 0.12 0.07 0.04 0.11 0.08 0.13 0.1±0.0 0.14 0.06 0.08 0.12 0.10 0.11 0.09 0.06 0.13 0.04 0.03 0.1±0.0 Mean Sand (%) Clay (%) Sal. (‰) 5.1 42.8 15.0 3.2 34.9 20.7 2.6 29.3 22.5 1.4 23.7 24.2 3.1±1.6 32.7±8.2 20.6±4.0 6.4 33.3 19.5 8.2 22.6 24.0 12.2 8.4 32.4 9.8 12.5 30.5 11.6 24.0 22.0 3.3 12.1 21.3 1.6 44.0 13.2 0.8 34.2 11.8 1.6 20.6 12.7 1.6 53.6 8.2 0.4 67.4 10.3 -0.2 65.6 7.3 -0.1 19.4 23.7 -0.1 53.1 11.9 4.1±4.6 33.6±20.0 17.8±8.1 0.4 24.8 20.7 0.4 11.7 23.3 1.0 8.3 30.7 1.0 27.2 19.6 2.0 20.5 12.9 3.0 1.7 25.7 6.5 20.6 27.2 3.9 6.6 22.6 2.3±2.1 15.2±9.3 22.8±5.4 2.5 14.9 23.9 6.0 32.9 12.8 7.0 55.8 7.3 14.5 19.9 25.4 19.9 22.3 26.1 23.4 18.9 29.1 20.3 16.8 28.2 17.2 30.8 17.8 16.0 12.7 23.9 19.3 63.2 8.6 14.0 42.5 17.9 14.5±6.7 30.1±17.1 20.1±7.7 ‘Gr.(n)’ shows the number (n) of samples in each clustering groups (Gr.). - 28 - Silt (%) WD (m) 42.2 2.1 44.4 2.4 48.2 2.3 52.1 2.1 46.7±4.4 2.2±0.1 47.2 2.5 53.5 10.5 59.2 3.8 57.0 5.0 53.9 2.1 66.6 2.2 42.8 4.0 54.0 3.2 66.7 5.0 38.2 2.2 22.3 2.2 27.1 1.8 56.9 3.5 35.0 3.8 48.6±13.8 3.7±2.2 54.5 2.3 65.0 2.9 61.0 3.2 53.2 5.2 66.6 4.1 72.7 7.1 52.2 3.5 70.8 6.0 62.0±8.0 4.3±1.7 61.2 7.1 54.3 4.4 37.0 8.0 54.7 2.8 51.6 5.0 52.0 4.0 55.0 5.8 51.4 4.8 63.4 15.0 28.2 18.0 39.5 16.0 49.8±10.7 8.3±5.4 Table 5 Results of the surface sediment (groups) and environmental variables (continued) Gr.(n) Sample ID Long (ºE) Lat(ºN) G5(22) PE 69 PE 70 PE 71 PE 72 PE 73 PE 02 PE 64 PE 57 PE 58 PE 62 PE 63 PE 75 PE 77 PE 65 PE 66 PE 67 PE 68 PE 74 PE 78 PE 79 PE 80 PE 81 113.72 113.77 113.78 113.75 113.75 113.69 113.65 113.65 113.68 113.68 113.66 113.68 113.68 113.62 113.60 113.64 113.68 113.70 113.60 113.65 113.70 113.60 22.34 22.37 22.38 22.39 22.35 22.50 22.38 22.45 22.46 22.58 22.45 22.49 22.49 22.33 22.29 22.31 22.32 22.36 22.25 22.25 22.25 22.19 PE 82 PE 83 PE 84 PE 85 PE 86 PE 87 PE 88 PE 89 PE 90 PE 91 PE 92 113.65 113.70 113.62 113.67 113.60 113.65 113.77 113.76 113.75 113.73 113.80 22.19 22.19 22.13 22.13 22.08 22.08 22.25 22.19 22.13 22.08 22.10 PE 29 PE 30 PE 31 PE 32 PE 33 PE 34 PE 39 PE 40 PE 35 PE 36 PE 37 PE 04 PE 38 114.08 114.12 114.02 113.96 114.10 113.98 113.93 113.96 113.95 113.90 113.90 113.83 113.89 22.23 22.25 22.26 22.22 22.32 22.35 22.46 22.48 22.36 22.32 22.38 22.46 22.43 PE 25 PE 26 PE 27 PE 28 114.27 114.23 114.18 114.08 22.24 22.21 22.22 22.19 PE 21 PE 22 PE 23 PE 24 114.45 114.45 114.45 114.32 22.37 22.29 22.22 22.20 Ave. G6 (11) Ave. G7(13) Ave. G8(4) Ave. G9(4) Ave. δ13C (‰) C/N -23.1 -23.6 -23.8 -22.0 -22.9 -23.7 -23.2 -22.7 -23.2 9.9 12.0 11.6 9.9 10.3 11.7 9.9 10.7 11.4 8.5 11.2 11.8 11.7 11.3 8.9 8.9 12.2 11.1 8.4 9.1 9.7 8.8 10.4±1.3 9.4 9.6 9.4 9.7 10.3 10.3 9.4 9.7 9.9 10.6 8.8 9.7±0.5 8.8 9.0 7.8 8.0 10.3 10.1 9.3 9.0 10.6 9.0 10.4 11.1 12.3 10.4±3.0 8.0 7.1 7.7 10.0 8.2±1.2 6.8 6.8 6.5 7.1 6.8±0.2 -23.5 -23.1 -22.3 -23.5 -22.9 -22.6 -23.4 -22.9 -23.1 -23.1 -23.4 -23.1 -23.1±0.4 -22.9 -23.2 -23.3 -22.9 -23.4 -23.0 -23.2 -23.2 -22.9 -23.1 -22.5 -23.1±0.2 -22.3 -23.2 -21.8 -21.9 -24.1 -24.1 -23.8 -23.9 -22.2 -22.8 -23.2 -23.9 -22.8 -23.1±0.9 -21.5 -21.0 -21.8 -22.5 -21.7±0.6 -21.0 -20.8 -21.2 -21.1 -21.0±0.2 TOC (%) TN(%) 1.23 1.38 0.95 0.96 1.13 1.69 1.27 1.35 1.26 0.25 1.21 1.11 1.11 0.71 0.94 1.07 1.13 1.38 1.24 1.25 1.26 1.24 1.1±0.3 1.13 1.16 1.23 0.90 1.50 0.93 1.09 1.10 1.09 0.91 0.98 1.1±0.2 1.02 0.83 1.13 0.62 0.59 0.78 1.05 1.23 1.45 0.39 0.56 0.90 0.75 0.9±0.3 0.57 0.68 0.92 1.60 0.9±0.5 0.68 0.75 0.63 0.92 0.7±0.1 0.12 0.12 0.08 0.10 0.11 0.14 0.13 0.13 0.11 0.03 0.11 0.09 0.10 0.06 0.11 0.12 0.09 0.12 0.15 0.14 0.13 0.14 0.1±0.0 0.12 0.12 0.13 0.09 0.15 0.09 0.12 0.11 0.11 0.09 0.11 0.1±0.0 0.12 0.09 0.14 0.08 0.06 0.08 0.11 0.14 0.07 0.04 0.05 0.08 0.06 0.1±0.0 0.07 0.09 0.12 0.09 0.1±0.0 0.10 0.11 0.10 0.13 0.1±0.0 Mean Sand (%) Sal. (‰) 20.8 12.5 24.1 15.7 24.0 15.8 17.9 16.8 20.3 18.4 11.9 24.6 13.5 36.0 10.7 13.3 12.0 12.5 11.0 37.8 13.1 9.3 11.0 26.9 10.9 23.5 14.1 12.8 13.8 22.0 15.5 7.1 18.5 19.9 17.0 16.2 17.6 20.3 19.9 9.0 22.1 13.7 20.3 16.1 16.4±4.4 18.2±7.9 23.0 16.9 25.9 14.7 24.3 15.4 27.4 19.6 28.8 17.1 30.0 20.9 25.4 16.0 28.5 14.8 29.4 18.6 30.7 16.9 30.9 15.9 27.7±2.7 17.0±2.0 31.3 15.8 32.1 2.6 30.4 6.8 31.4 8.3 31.5 29.0 30.3 18.9 24.9 4.0 20.3 13.8 30.5 13.9 30.2 24.9 29.8 47.8 26.0 35.7 29.8 11.9 29.1±3.4 18.0±13.2 33.5 21.3 32.4 16.5 32.4 9.9 31.1 23.5 32.4±1.0 17.8±6.0 33.3 17.3 33.4 14.4 33.5 19.8 33.5 3.4 33.4±0.1 13,7±7.2 Clay (%) Silt (%) WD (m) 33.3 31.3 28.4 30.5 28.9 28.6 11.1 29.8 29.2 11.2 27.0 20.8 17.8 34.5 31.1 38.3 18.4 33.0 33.2 32.5 35.2 31.3 28.0±7.4 28.2 29.7 29.5 25.2 28.7 20.2 30.2 25.6 24.3 19.8 21.5 25.7±3.9 24.8 31.0 32.8 15.8 21.7 24.9 32.3 23.3 29.3 22.4 15.2 21.5 28.2 24.9±5.7 17.6 17.4 26.0 18.1 19.8±4.1 18.8 21.6 24.6 27.8 23.2±3.9 ‘Gr.(n)’ shows the number (n) of samples in each clustering groups (Gr.). - 29 - 54.1 13.5 53.0 20.0 55.8 13.0 52.8 15.0 52.7 15.0 46.8 6.9 53.0 7.0 57.0 7.0 58.3 10.0 51.0 8.0 63.7 5.5 52.3 7.0 58.7 5.4 52.7 5.0 46.9 3.5 54.6 13.0 61.6 12.0 50.8 9.0 46.5 6.2 58.6 5.3 51.1 5.8 52.6 5.3 53.8±4.5 9.0±.4.3 54.9 7.0 55.6 6.0 55.1 6.8 55.2 8.5 54.2 6.0 58.9 8.5 53.8 6.3 59.6 8.5 57.1 8.5 63.3 11.5 62.6 14.0 57.3±3.3 8.3±2.5 59.4 8.0 66.5 35.0 60.4 8.0 75.9 6.0 49.2 20.0 56.2 11.0 63.8 4.0 62.9 3.0 56.8 14.0 52.6 5.0 37.0 20.0 42.8 4.8 59.9 8.0 57.2±10.1 11.3±9.1 61.1 21.0 66.1 14.0 64.1 14.0 58.4 14.0 62.4±3.4 15.8±3.5 63.9 24.0 63.9 25.0 55.6 28.0 68.7 31.0 63.0±5.5 27.0±3.2 Table 6 Spatial variation of the organic carbon signature and environmental conditions within the Pearl River estuary Environment δ13C (‰) C/N TOC (%) TN (%) Mean Sal. Sand (%) Clay (%) Silt (%) WD (m) (‰) Freshwater -23.8±1.5 12.7±2.3 1.2±0.7 0.1±0.0 3.4±3.6 27.8±17.7 19.8±7.0 52.4±12.7 3.7±2.0 (G1-G3) Fresh-brackish -23.4±0.8 11.6±2.5 1.1±0.3 0.1±0.0 15.8±5.3 22.1±12.8 25.3±8.3 52.5±7.2 8.8±4.6 (G4-G5) Marine-brackish -23.1±0.6 9.7±1.0 1.0±0.3 0.1±0.0 28.4±3.1 17.5±9.7 25.3±4.9 57.2±7.7 9.9±6.9 (G6-G7) Marine -21.4±0.5 7.5±1.1 0.8±0.3 0.1±0.0 32.9±0.8 15.8±6.5 21.5±4.2 62.7±4.2 21.4±6.8 (G8-G9) - 30 - Figures Fig. 1 - 31 - Fig. 2 - 32 - -10.0 a δ13 C(‰) -15.0 Average value General C4 plants Sugarcane (C4) General C3 plants Agricultural C3 plants Mangroves (C3) C4 plants (24.6, -13.1) -20.0 -25.0 -30.0 C3 plants (22.7, -29.0) -35.0 0.0 -10.0 10.0 20.0 40.0 b 50.0 60.0 70.0 Sugarcane (C 4 ) General C4 plants -15.0 δ13 C(‰) 30.0 -20.0 -25.0 Agricultural C3 plants Mangroves (C 3 ) -30.0 General C3 plants -35.0 0.0 10.0 20.0 30.0 C/N Fig. 3 - 33 - 40.0 50.0 -18.0 a Agricultural soil Riverbank soil -20.0 Mangrove soil Forest soil δ13C(‰) -22.0 -24.0 -26.0 -28.0 -30.0 6.0 -20.0 8.0 10.0 12.0 14.0 16.0 18.0 20.0 22.0 b Agricultural soil -22.0 Mangrove soil -24.0 δ 13C(‰) Riverbank soil -26.0 Forest soil -28.0 -30.0 +6.0 +8.0 +10.0 +12.0 +14.0 +16.0 C/N Fig. 4 - 34 - +18.0 +20.0 +22.0 -21.0 Winter Summer -22.0 -23.0 δ 13C(‰) -24.0 -25.0 -26.0 -27.0 -28.0 -29.0 5.0 6.0 7.0 8.0 C/N Fig. 5 - 35 - 9.0 10.0 11.0 - 36 - -21.0 Winter δ13 C - C/N -22.0 G6 Winter δ13 C - salinity G5 -24.0 G4 -25.0 G4 -25.0 G3 G3 -26.0 c G6 -23.0 G5 -24.0 -26.0 -27.0 -27.0 G2 G2 -28.0 -28.0 -29.0 -29.0 5.0 6.0 7.0 -21.0 8.0 9.0 10.0 Summer δ13 C - C/N 11.0 b -22.0 0.0 5.0 10.0 -21.0 15.0 20.0 25.0 30.0 35.0 Summer δ13 C - salinity 40.0 d -22.0 -23.0 -23.0 G3 -24.0 δ13C(‰) -21.0 -22.0 -23.0 δ13C(‰) a G5 G2 -25.0 -27.0 -28.0 -28.0 -29.0 -29.0 5.0 6.0 7.0 8.0 9.0 G4 -26.0 -27.0 10.0 11.0 G5 G2 -25.0 G4 -26.0 G3 -24.0 0.0 5.0 10.0 15.0 20.0 25.0 Salinity (PSU) C/N Fig. 6 - 37 - 30.0 35.0 40.0 -22.0 -23.0 δ13 C(‰) -24.0 9.5, -24.3 -25.0 10.7, -25.6 -26.0 Summer POC on NET Summer POC on filterpaper -27.0 Group average -28.0 4.0 6.0 8.0 10.0 12.0 C/N Fig. 7 38 14.0 16.0 18.0 -20.0 a G9 G8 G7 G6 G5 G4 G3 G2 G1 -21.0 -22.0 δ13C(‰) -23.0 -24.0 -25.0 -26.0 -27.0 -28.0 6.0 8.0 10.0 12.0 14.0 16.0 18.0 -20.0 20.0 b G9 -21.0 G8 -22.0 δ13C(‰) G7 G5 G3 -23.0 G6 G4 -24.0 G2 -25.0 G1 -26.0 -27.0 6.0 8.0 10.0 12.0 14.0 C/N Fig. 8 39 16.0 18.0 20.0