Proper Use and Care of the Micropipettors
advertisement

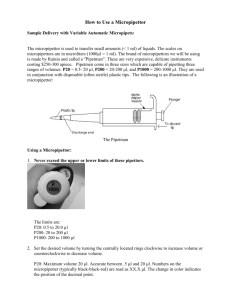
NAME ___________________________ LAB PROPER USE & CARE OF MICROPIPETTORS (Modified from Joslin/Boulay) Since we will be measuring tiny amounts of reagents in the next few labs, it will be important to become familiar with the metric measure known as the microliter (l). The plastic pipettors we have used so far have been effective at measuring 1 milliliter (ml), and even 0.5 ml & 0.25 ml. When manipulating DNA, RNA, enzymes & other macromolecules, it will be important to measure even smaller amounts. The micropipettor does this very effectively, but only if used correctly. In this introductory lab, you will have the opportunity to measure small amounts of liquid - as you would during an actual lab - and then check to see how accurate your micropipetting techniques are. It will be important to be able to convert ml to l. 1 Liter = 1000 ml 1 ml = 1000 l To convert ml to l, simply MULTIPLY by 1000 To convert l to ml, simply DIVIDE by 1000 Try the following conversions: 10 l = _____ ml 100 l = _____ ml 0.025 ml = _____ l We will use a pipettor that measures from 0.002 ml - 0.020 ml. What is this range in l? ____________________ I. Use of the Micropipettor The "NEVERS" Never rotate the volume adjuster beyond the upper OR lower range of the pipet as stated by the manufacturer. Never use the micropipettor without a tip in place. Never lay down the micropipettor with a filled tip. Never let the plunger "snap" back up. Never immerse the barrel of the micropipettor in fluid. 0.75 ml = _____ l II. Parts & Functions of the Micropipettor III. Reading the Volume (For a 2-20 l Micropipettor) 1. Rotating the volume adjuster clockwise decreases the desired volume. 2. Rotating the volume adjuster counter-clockwise increases the desired volume. 3. What display would measure 10.0 l? 4. For this specific micropipette, never rotate below ____ l or above ____ l. IV. Pipetting Directions 1. Rotate the volume adjuster to the desired setting. Note the change in plunger length as the volume is changed. Be sure to locate the decimal point properly when reading the volume setting. 2. Firmly seat a disposable tip on the end of the pipettor. 3. When withdrawing or expelling fluid, always hold the tube firmly between the thumb & forefinger. Hold the tube nearly at EYE LEVEL to observe the change in fluid in the pipet tip. DO NOT pipet with the tube in the test tube rack OR have another person HOLD the tube while pipetting. 4. Each tube must be held properly during each manipulation. Grasping the tube body, rather than the lid, provides more control and avoids contamination from the lid. 5. Hold the pipettor almost VERTICAL when withdrawing or expelling fluid. Always pipet FROM the reagent tube TO the reaction tube. 6. Your pipettor has a two-position plunger with friction “stops.” Depressing to the 1st stop measures the desired volume. Depressing to the 2nd stop introduces an additional volume of air to blow out any solution remaining in the tip. NOTE: Depressing to the 2nd stop is only done when expelling fluid. Pay attention to these friction stops, which can be felt with the thumb. 7. To WITHDRAW Fluid from a Reagent Tube a) Depress the plunger to the 1st stop & HOLD this position. The tip should NOT be in any fluid yet. Now dip the tip into the reagent tube containing the solution to be withdrawn. Draw fluid into the tip by gradually releasing the plunger. b) Check that there is no air space at the very end of the tip. 8. To EXPEL Sample into a Reaction Tube a) Touch the pipet tip to the inside wall of the reaction tube into which the sample will be emptied. This creates a capillary effect that helps draw fluid out of the tip. b) Slowly depress the plunger to the 1st stop to expel the sample. Depress to the 2nd stop to blow out the last bit of fluid. HOLD the plunger in this depressed position. c) Slide the pipet out of the reaction tube with the plunger still depressed. This will avoid sucking any liquid back into tip. Gradually release the plunger when the pipet is completely out of the reaction tube. d) When you are done, eject the tip into the waste cup by pressing the ejector button. 9. To Prevent Cross-Contamination a) Add the appropriate amounts of a single solution to ALL the reaction tubes in a row, and then change the tip for the next solution. b) Use a fresh tip for each NEW solution to be pipetted. c) If contamination occurs, change the tip. V. The Microcentrifuge The microcentrifuge is used to separate substances according to density, but it can also be used to mix together very small amounts of reagents in a test tube. To mix the contents together in your reaction tubes, place the tubes in a balanced configuration in the microcentrifuge. Apply a 5-second pulse. Spinning the tubes in an unbalanced position will damage the motor. PROCEDURE This exercise simulates setting up a reaction using a micropipettor with a range of 2 - 20 l. Each group will complete the following: 1. Use permanent marker to label 3 reaction tubes A, B & C. 2. Use the matrix on the next page as a checklist while adding solutions to each reaction tube. 3. Set the micropipettor to 4 l. ADD Solution I (Sol I) to EACH reaction tube (A, B & C). TUBE Sol I Sol II Sol III Sol IV A 4 l 5 l 2 l --- B 4 l 5 l --- 2 l C 4 l 3 l 2 l 2 l 4. Use a fresh tip to add the appropriate volume of Solution II (Sol II) to a "clean" spot in each reaction tube (A, B & C). 5. Use a fresh tip to add 2 l of Solution III (Sol III) to tubes A & C. 6. Use a fresh tip to add 2 l of Solution IV (Sol IV) to tubes B & C. 7. Close the lids. Pool the reagents together by placing the tubes in a microcentrifuge in a balanced configuration and applying a short, 5-second pulse. 8. A total of 11 l of reagents was added to each reaction tube. To check that your measurements were accurate, set the pipette to 11 l and very carefully withdraw the solution from EACH tube. a) Is the tip just filled? A ______ B ______ C ______ b) Is there a small volume left in the tube? A ______ B ______ C ______ c) After extracting all the fluid from the tube, is an air space left in the tip end? A ______ B ______ C ______ This air can be displaced - and the actual volume determined - by simply rotating the volume adjustment to push fluid to the very end of the tip. Then, read the volume directly. 9. If 2 out of 3 of your measurements were inaccurate, repeat the exercise to obtain near perfect results. RESULTS & DISCUSSION 1. Why must the tubes be balanced in the microcentrifuge? ------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------- 2. What are 2 functions of a centrifuge? ------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------3. Explain when it's okay to use the same micropipette tip AND when it's not okay to use the same micropipette tip. ------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------- 4. What are 2 common errors in handling a micropipettor that can account for over-pipetting (i.e., pipetting too much reagent into a reaction tube)? ------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------- 5. What are 2 common errors in handling a micropipettor that can account for under-pipetting (i.e., pipetting too little reagent from a reagent tube)? -------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------