Summary Category: PROTEINS & PEPTIDES Characterization Of
advertisement

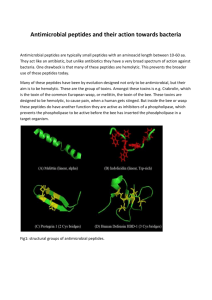
Summary Category: PROTEINS & PEPTIDES Characterization Of Antimicrobial Peptides From The Venom Of The Spider Lycosa erythrognatha (Lucas, 1836) Daniel Moreira Santos1; Frederic Frezard2; Jarbas Magalhães Resende3; Dorila Piló-Veloso3; Luiz Macêdo Farias4; Maria Auxiliadora Roque Carvalho4; Adriano Monteiro Castro Pimenta1; Maria Elena De Lima1. santosdm@gmail.com Lab. Venenos e Toxinas Animais, Depto. Bioquímica e Imunologia, ICB; Lab. Biofísica De Nanossistemas Lipídicos, Depto. Fisiologia E Biofísica, ICB; 3Lab. Ressonância Nuclear Magnética, Depto. Química, ICEX; 4Lab. Anaeróbios, Depto. Microbiologia, ICB, UFMG, Belo Horizonte - MG - Brazil. 1 2 Introduction: A promising class of venom’s molecules is the antimicrobial peptides. The dramatic increase on frequency of opportunistic bacterial infections has prompted the search for an arsenal diversification of antimicrobial agents. The objective of this work was to purify, synthesize and characterize the structure and activity of antimicrobial peptides from the venom of the spider Lycosa erythrognatha. Methods: The purification of the peptides was performed in the HPLC system using cation exchange chromatography (CM-2SW column, Tosoh), followed by reverse phase chromatography (C8 Column, Supelco). Mass analysis was performed in MALDI-ToFToF and ESI-Q-ToF. The peptides were sequenced using the automated Edman degradation and mass spectrometry (MS-MS). The peptides were synthesized using the Fmoc/t-butila methodology followed by purification in reverse phase chromatography. The antimicrobial activity was tested in microplates against Escherichia coli (ATCC 25922) and Staphylococcus aureus (ATCC 33591). The effect of peptides on lipid membranes was investigated using liposomes consisting of phosphatidylcholine. The tertiary structure of peptides was determined by nuclear magnetic resonance (NMR). Results: The obtained peptides comprise 207 different molecular weights. 25 % of these peptides shown mass between 900 and 3500 Da and 75 % shown mass between 3500 and 9800 Da. Three of these peptides, named LyeTx I, II and III were sequenced and showed masses of 2831 Da, 1941 Da and 2065 Da, respectively. They presented similar sequences to antimicrobial peptides. The three peptides were synthesized and then analyzed by mass spectrometry and showed a high degree of purity. Antimicrobial assays of LyeTx I and II demonstrated activities at μmol L-1 concentration, inhibiting the growth of E. coli and S. aureus. The lytic activity was measured by the release of calcein from liposomes. The minimal detected activity was in the range of pmol L-1 for peptides LyeTx I and II. The dose response curves for the liposome membrane permeabilization, by these peptides, were dose-dependent. The tertiary structures of these peptides were determined by NMR and showed an amphipathic alpha-helix structure, present in most of the known antimicrobial peptides. Conclusion: The synthetic peptides LyeTx I and II showed activity against Gram positive and Gram negative bacteria and its structure was determined by NMR. These peptides can serve as models for developing of new antibiotics. Financial support: CNPq, FAPEMIG, CAPES e INCTTOX