SJ #11 “Temperature and Density Notes”

SJ #12 “Temperature and Density”
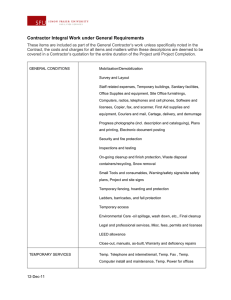
Lesson 5 p.43
* TEMPERATURE : the amount of ENERGY that the molecules in a substance have.
-measured with a thermometer
“therm” = heat
“meter” = measure
-EX: high temp. fast moving,
high energy molecules
low temp. slow moving,
low energy molecules
* HEAT : the TRANSFER of ENERGY from a hotter object to a colder object. (“transfer” means to move)
--add heat temp. increases
--remove heat temp. decreases p. 43 “Changing Temperature, Changing Density”
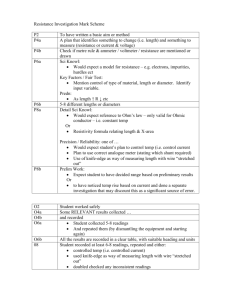
-MOST matter increases in VOLUME when it gets hotter EXPANSION
*EX: if you heat a gas, particles will expand, but MASS STAYS THE SAME
gas will be LESS DENSE
-MOST matter decreases in VOLUME when it gets colder, CONTRACTION
*EX: if you cool a gas, particles will contract, but MASS STAYS THE SAME
gas will be MORE DENSE
**WATER IS AN EXCEPTION!
**H
2
O actually expands when it freezes, so it becomes less dense and floats.
**This is why pipes burst a lot in winter.
**Other exceptions: gallium, bismuth, acetic acid, antimony, and silicon, due to their molecular geometry. They form “tetrahedral lattices”, a fancy term for a kind of crystal.
*Eureka! Episodes 19, 20 & 21
*Expansion & Contraction
1)
2)
3)
*Measuring Temperature
1)
2)
3)
*Temperature vs. Heat
1)
2)
3)
+ 1 drawing
*Read p. 4445 “Measuring Temperature By Degrees”
--write 10 facts
*3 from p. 44
*7 from p. 45
*What is the most interesting idea you read in this article? Answer in a complete sentence!